Abstract
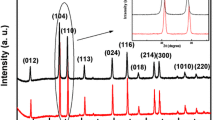
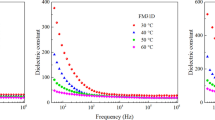
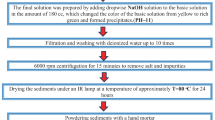
The present study describes the green synthesis of hematite (α-Fe2O3) nanomaterials using the Tabernaemontana divaricata flower extract as a reducing/cap**/stabilizing agent and the changes observed in the structural, optical, magnetic and dielectric properties of the product with respect to the pristine counterpart. Powder XRD study showed that use of the extract in green synthesis produces hematite nanomaterials of reduced size, whereas FE-SEM, HRTEM and EDX studies revealed the morphology, particle size distribution, crystallinity as well as the elemental contributions. FT-IR spectroscopy detected the different functional groups and metal-ion bonding present in the synthesized nanomaterials. Raman study observed the seven Raman active (two A1g and five Eg) modes with different intensities. From the UV–Vis study, different electronic transitions occurred in the hematite nanomaterials were identified, and the direct (1.90–1.97 eV) and indirect (1.21–1.46 eV) energy band gap values were estimated. Two photoluminescence peaks (one in visible and the other in near infrared region) detected in the spectra indicated the presence of various defect levels in the hematite nanoparticles within the energy band gap. Magnetic measurements exhibited the Morin transition to occur in these materials, while Mössbauer spectra successfully showed the magnetic contribution of the iron atoms occupying the core and the surface parts of the nanoparticles. Use of the extract in green synthesis produced hematite nanomaterials with extremely high dielectric constant (~ 105) and significantly lower dielectric loss at low frequency and at room temperature when compared to the pristine one which signify their huge application potential. The results obtained from the characterization studies made on the pristine and green synthesized hematite nanomaterials revealed that the green synthesis of hematite nanoparticles using Tabernaemontana divaricata flower extract can efficiently modify their optical, magnetic and dielectric properties. Thus, using commercially cheap starting materials and extracts made from naturally available plant parts, materials having huge application potentials (like microwave and energy storage appliances) can be synthesized at low-cost by the simple green synthesis approach.














Similar content being viewed by others
Data availability
Data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
S. Dua, H. Ahmed, N. Arfin, Soft nanomaterials and their applications, in Nanomaterials: the building blocks of modern technology: synthesis, properties and applications. ed. by T. Khan, M. Jawaid, K.A. Ahmad, B. Singh (Springer Nature Singapore, Singapore, 2023), pp.27–68. https://doi.org/10.1007/978-981-99-4149-0_3
M.S. Chavali, M.P. Nikolova, Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 1(6), 607 (2019)
S. Iravani, Bio-based synthesis of magnetic nanoparticles and their applications, in Magnetic nanostructures, nanotechnology in the life sciences. ed. by K. A. A.-E. et al. (Springer Nature Switzerland AG, Switzerland, 2019), pp.13–31. https://doi.org/10.1007/978-3-030-16439-3_2
F. Bødker, M.F. Hansen, C.B. Koch, K. Lefmann, S. Mørup, Magnetic properties of hematite nanoparticles. Phys. Rev. B 61(10), 6826 (2000)
Y.R. Kumar, S. Kavita, A. Palanisamy, M. Vasundhara, Structural optical and magnetic properties of chitosan mediated α-Fe2O3 nanoparticles. Mater. Today 99(2), 1064–1069 (2023)
A.A. Qureshi, S. Javed, H.M.A. Javed, M. Jamshaid, U. Ali, M.A. Akram, Systematic investigation of structural, morphological, thermal, optoelectronic, and magnetic properties of high-purity hematite/magnetite nanoparticles for optoelectronics. Nanomaterials 12(10), 1635 (2022)
P. Dhar, R. Vinu, Microwave-assisted catalytic solvolysis of lignin to phenols: kinetics and product characterization. ACS Omega 3(11), 15076–15085 (2018)
P. Kumar, S. Kumar, N. Thakur, Azadirachta indica and polyvinylpyrrolidone encapsulated Fe2O3 nanoparticles to enhance the photocatalytic and antioxidant activity. Inorgan. Chem. Commun. 155, 111084 (2023)
Y. Li, J. Dang, Y. Ma, H. Ma, Hematite: a good catalyst for the thermal decomposition of energetic materials and the application in nano-thermite. Molecules 28(5), 2035 (2023)
Y. Ling, G. Wang, J. Reddy, C. Wang, J.Z. Zhang, Y. Li, The influence of oxygen content on the thermal activation of hematite nanowires. Angew. Chem. 124(17), 4150–4155 (2012)
M.V. Reddy et al., α-Fe2O3 nanoflakes as an anode material for Li-ion batteries. Adv. Func. Mater. 17(15), 2792–2799 (2007)
K.G. Gareev, Diversity of iron oxides: mechanisms of formation, physical properties and applications. Magnetochemistry 9(5), 119 (2023)
S. Kumar, M. Kumar, A. Singh, Synthesis and characterization of iron oxide nanoparticles (Fe2O3, Fe3O4): a brief review. Contemp. Phys. 62(3), 144–164 (2021). https://doi.org/10.1080/00107514.2022.2080910
M. Muhajir, P. Puspitasari, J.A. Razak, Razak, Synthesis and applications of Hematite α-Fe2O3: A review. J. Mech. Eng. Sci. Technol. (JMEST) 3(2), 51–58 (2020)
B. Wang, Q. Wei, S. Qu, Synthesis and characterization of uniform and crystalline magnetite nanoparticles via oxidation-precipitation and modified co-precipitation methods. Int. J. Electrochem. Sci. 8(3), 3786–3793 (2013)
M.A. Malik, M.Y. Wani, M.A. Hashim, Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 5(4), 397–417 (2012)
L. Zhuang, W. Zhang, Y. Zhao, H. Shen, H. Lin, J. Liang, Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Sci. Rep. 5(1), 1–6 (2015)
S. Kundu, M. Chakraborty, A. Bhattacharjee, Solid-state reaction of ferrocene controlled by co-precursor and reaction atmosphere leading to hematite and cohenite nanomaterials: a reaction kinetic study. J. Phys. Chem. C 127(37), 18397–18408 (2023). https://doi.org/10.1021/acs.jpcc.3c04772
M.S. Islam, Y. Kusumoto, M. Abdulla-Al-Mamun, Novel rose-type magnetic (Fe3O4, γ-Fe2O3 and α-Fe2O3) nanoplates synthesized by simple hydrothermal decomposition. Mater. Lett. 66(1), 165–167 (2012)
Q.-X. Gao, X.-F. Wang, J.-L. Di, X.-C. Wu, Y.-R. Tao, Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with 110 and 001 planes for methane combustion and CO oxidation. Catal. Sci. Technol. 1(4), 574–577 (2011)
X. Wang et al., Synthesis of β-FeOOH and α-Fe2O3 nanorods and electrochemical properties of β-FeOOH. J. Mater. Chem. 14(5), 905–907 (2004)
S. Iravani, Green synthesis of metal nanoparticles using plants. Green Chem. 13(10), 2638–2650 (2011). https://doi.org/10.1039/c1gc15386b
D. Jagwani, P. Hari Krishna, Nature’s nano-assets: Green synthesis, characterization techniques and applications—a graphical review. Mater. Today Proc. 46, 2307–2317 (2021). https://doi.org/10.1016/j.matpr.2021.04.185
M. Huston, M. Debella, M. Dibella, A. Gupta, Green synthesis of nanomaterials. Nanomaterials 11(8), 1–29 (2021). https://doi.org/10.3390/nano11082130
A. Rana, K. Yadav, S. Jagadevan, A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 272, 122880 (2020). https://doi.org/10.1016/j.jclepro.2020.122880
X. Zhang, Applications of kinetic methods in thermal analysis: a review. Eng. Sci. 14(2), 1–13 (2020)
Y. Shao et al., Green synthesis of multifunctional fluorescent carbon dots from mulberry leaves (Morus alba L.) residues for simultaneous intracellular imaging and drug delivery. J. Nanopart. Res. 22(8), 1–11 (2020)
B. Mohapatra, D. Kumar, N. Sharma, S. Mohapatra, Morphological, plasmonic and enhanced antibacterial properties of Ag nanoparticles prepared using Zingiber officinale extract. J. Phys. Chem. Solids 126, 257–266 (2019). https://doi.org/10.1016/j.jpcs.2018.11.020
R. Banasiuk et al., Carnivorous plants used for green synthesis of silver nanoparticles with broad-spectrum antimicrobial activity. Arab. J. Chem. 13(1), 1415–1428 (2020)
T. Sinha, M. Ahmaruzzaman, A novel green and template free approach for the synthesis of gold nanorice and its utilization as a catalyst for the degradation of hazardous dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 142, 266–270 (2015)
T. Wang, X. **, Z. Chen, M. Megharaj, R. Naidu, Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total. Environ. 466, 210–213 (2014)
I. Ali, Water photo splitting for green hydrogen energy by green nanoparticles. Int. J. Hydrogen Energy 44(23), 11564–11573 (2019)
V. Kumar, D.K. Singh, S. Mohan, D. Bano, R.K. Gundampati, S.H. Hasan, Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion. J. Photochem. Photobiol. B. 168, 67–77 (2017)
M. Krishnan et al., Antifouling assessments on biogenic nanoparticles: a field study from polluted offshore platform. Mar. Pollut. Bull. 101(2), 816–825 (2015)
S. Haseena et al., Bio-synthesize of photocatalytic Fe2O3 nanoparticles using Leucas aspera and Jatropha podagrica leaf extract for an effective removal of textile dye pollutants. Optik 249, 168275 (2022)
J. Yoonus, R. Resmi, B. Beena, valuation of antibacterial and anticancer activity of green synthesized iron oxide (α-Fe2O3) nanoparticles. Mater. Today 46, 2969–2974 (2021)
S.K. Noukelag, C.J. Arendse, M. Maaza, Biosynthesis of hematite phase α-Fe2O3 nanoparticles using an aqueous extract of Rosmarinus officinalis leaves. Mater. Today 43, 3679–3683 (2021)
K. Velsankar, G. Parvathy, S. Mohandoss, M. Krishna Kumar, S. Sudhahar, Celosia argentea leaf extract-mediated green synthesized iron oxide nanoparticles for bio-applications. J. Nanostructure Chem. 12(4), 625–640 (2022)
M.S.H. Bhuiyan et al., Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 6(8), e04603 (2020)
S.O. Aisida et al., Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanosci. 10(1), 305–315 (2020). https://doi.org/10.1007/s13204-019-01099-x
M. Jamzad, M. Kamari Bidkorpeh, Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. J. Nanostructure Chem. 10(3), 193–201 (2020). https://doi.org/10.1007/s40097-020-00341-1
R. Vinayagam, S. Pai, T. Varadavenkatesan, M.K. Narasimhan, S. Narayanasamy, R. Selvaraj, Structural characterization of green synthesized α-Fe2O3 nanoparticles using the leaf extract of Spondias dulcis. Surfaces Interfaces 20, 100618 (2020). https://doi.org/10.1016/j.surfin.2020.100618
N. Madubuonu et al., Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A 126(1), 72 (2020). https://doi.org/10.1007/s00339-019-3249-6
A. Bouafia, S.E. Laouini, Green synthesis of iron oxide nanoparticles by aqueous leaves extract of Mentha Pulegium L.: Effect of ferric chloride concentration on the type of product. Mater. Lett. 265, 127364 (2020). https://doi.org/10.1016/j.matlet.2020.127364
S. P. Rajendran and K. Sengodan, Synthesis and characterization of zinc oxide and iron oxide nanoparticles using Sesbania grandiflora leaf extract as reducing agent. J. Nanosci. 2017, (2017). https://doi.org/10.1155/2017/8348507
S. Naz, M. Islam, S. Tabassum, N.F. Fernandes, E.J.C. de Blanco, M. Zia, Green synthesis of hematite (α-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J. Mol. Struct. 1185, 1–7 (2019)
B.A. Abbasi, J. Iqbal, T. Mahmood, A. Qyyum, S. Kanwal, Biofabrication of iron oxide nanoparticles by leaf extract of Rhamnus virgata: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Appl. Organomet. Chem. 33(7), e4947 (2019)
T. Ahmad, R. Phul, N. Khatoon, M. Sardar, Antibacterial efficacy of Ocimum sanctum leaf extract-treated iron oxide nanoparticles. New J. Chem. 41(5), 2055–2061 (2017). https://doi.org/10.1039/C7NJ00103G
A.T. Khalil, M. Ovais, I. Ullah, M. Ali, Z.K. Shinwari, M. Maaza, Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 10(4), 186–201 (2017)
D. Mukherjee, S. Ghosh, S. Majumdar, K. Annapurna, Green synthesis of α-Fe2O3 nanoparticles for arsenic (V) remediation with a novel aspect for sludge management. J. Environ. Chem. Eng. 4(1), 639–650 (2016)
M. Alagiri, S.B.A. Hamid, Green synthesis of α-Fe2O3 nanoparticles for photocatalytic application. J. Mater. Sci. 25(8), 3572–3577 (2014)
N. Ndou, T. Rakgotho, M. Nkuna, I.Z. Doumbia, T. Mulaudzi, R.F. Ajayi, Green synthesis of iron oxide (Hematite) nanoparticles and their influence on sorghum bicolor growth under drought stress. Plants 12(7), 1425 (2023)
C. Sudhakar, M. Poonkothai, T. Selvankumar, K. Selvam, Facile synthesis of iron oxide nanoparticles using Cassia auriculata flower extract and accessing their photocatalytic degradation and larvicidal effect. J. Mater. Sci. 33(14), 11434–11445 (2022)
F. Buarki, H. AbuHassan, F. Al Hannan, F. Z. Henari, Green synthesis of iron oxide nanoparticles using Hibiscus rosa sinensis flowers and their antibacterial activity. J. Nanotechnol. 2022, (2022). https://doi.org/10.1155/2022/5474645
M.S. Aida, N. Alonizan, B. Zarrad, M. Hjiri, Green synthesis of iron oxide nanoparticles using Hibiscus plant extract. J. Taibah Univ. Sci. 17(1), 2221827 (2023)
D. Hassan et al., Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): their physical properties and novel biological applications. Artificial Cells Nanomed Biotechnol. 46(sup1), 693–707 (2018)
E. Rostamizadeh, A. Iranbakhsh, A. Majd, S. Arbabian, I. Mehregan, Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J Nanostruct. Chem. 10(2), 125–130 (2020). https://doi.org/10.1007/s40097-020-00335-z
F. Mohamed, M. Rabia, M. Shaban, Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Market. Res. 9(3), 4255–4271 (2020)
H.R. Ali, H.N. Nassar, N.S. El-Gendy, Green synthesis of α-Fe2O3 using Citrus reticulum peels extract and water decontamination from different organic pollutants. Energy Sources Part A 39(13), 1425–1434 (2017). https://doi.org/10.1080/15567036.2017.1336818
V.A. Niraimathee, V. Subha, R.S.E. Ravindran, S. Renganathan, Green synthesis of iron oxide nanoparticles from Mimosa pudica root extract. Int. J. Environ. Sustain. Dev. 15(3), 227–240 (2016)
T.S. Kam, H.S. Pang, Y.M. Choo, K. Komiyama, Biologically active ibogan and vallesamine derivatives from Tabernaemontana divaricata. Chem. Biodivers. 1(4), 646–656 (2004). https://doi.org/10.1002/cbdv.200490056
M.T. Sebastian, R. Ubic, H. Jantunen, Low-loss dielectric ceramic materials and their properties. Int. Mater. Rev. 60(7), 392–412 (2015)
T. Sarkar, S. Kundu, G. Ghorai, P.K. Sahoo, A. Bhattacharjee, Structural, spectroscopic and morphology studies on green synthesized ZnO nanoparticles. Adv. Nat. Sci. 14(3), 35001 (2023)
J.S. Justus, S.D.D. Roy, A.M.E. Raj, Synthesis and characterization of hematite nanopowders. Mater. Res. Express 3(10), 105037 (2016)
F. Wang, X.F. Qin, Y.F. Meng, Z.L. Guo, L.X. Yang, Y.F. Ming, Hydrothermal synthesis and characterization of α-Fe2O3 nanoparticles. Mater. Sci. Semicond. Process. 16(3), 802–806 (2013)
N. Chamkouri, N. Jomehzadeh, N. Naserzadeh, Rapid biosynthesis and antibacterial activity of zinc oxide nanoparticles using fruit peel of Punica granatumL as cellulose. Curr. Res. Green Sustain. Chem 2023, 100366 (6AD)
A. Kulshreshtha, J. Saxena, Alkaloids and Non Alkaloids of Tabernaemontana divaricata. Int. J. Res. Rev. 6(8), 2454–2237 (2019), [Online]. www.ijrrjournal.com
P. Salgado Mendoza, K. Márquez, O. Rubilar, D. Contreras, The effect of phenolic compounds on the green synthesis of iron nanoparticles (FexOy-NPs) with photocatalytic activity. Appl. Nanosci. 9, 371–385 (2018). https://doi.org/10.1007/s13204-018-0931-5
J.C. Kuriacose, S.S. Jewur, Studies on the surface interaction of acetic acid on iron oxide. J. Catal. 50(2), 330–341 (1977). https://doi.org/10.1016/0021-9517(77)90042-2
A. Miri, H. Najafzadeh, M. Darroudi, M.J. Miri, M.A.J. Kouhbanani, M. Sarani, Iron oxide nanoparticles: biosynthesis, magnetic behavior, cytotoxic effect. ChemistryOpen 10(3), 327–333 (2021). https://doi.org/10.1002/open.202000186
S.P.S. Porto, R.S. Krishnan, Raman effect of corundum. J. Chem. Phys. 47(3), 1009–1012 (1967)
L. Chen et al., Continuous shape-and spectroscopy-tuning of hematite nanocrystals. Inorg. Chem. 49(18), 8411–8420 (2010)
I. Chamritski, G. Burns, Infrared-and Raman-active phonons of magnetite, maghemite, and hematite: a computer simulation and spectroscopic study. J. Phys. Chem. B 109(11), 4965–4968 (2005)
I. R. Beattie and T. R. Gilson, The single-crystal Raman spectra of nearly opaque materials. Iron(III) oxide and chromium(III) oxide. j. Chem. Soc. A 980–986 (1970). https://doi.org/10.1039/J19700000980
I. Chourpa et al., Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 130(10), 1395–1403 (2005)
S. Kundu et al., Study on co-precursor driven solid state thermal conversion of iron(III)citrate to iron oxide nanomaterials. Appl. Phys. A 129(4), 264 (2023). https://doi.org/10.1007/s00339-023-06559-4
A. Rufus, N. Sreeju, V. Vilas, D. Philip, Biosynthesis of hematite (α-Fe2O3) nanostructures: size effects on applications in thermal conductivity, catalysis, and antibacterial activity. J. Mol. Liq. 242, 537–549 (2017)
B. Ahmmad et al., Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv. Powder Technol. 24(1), 160–167 (2013)
Y.P. He et al., Size and structure effect on optical transitions of iron oxide nanocrystals. Phys. Rev. B 71(12), 125411 (2005)
D.M. Sherman, T.D. Waite, Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Miner. 70(11–12), 1262–1269 (1985)
A. Lassoued et al., Synthesis, structural, optical, morphological and magnetic characterization of copper substituted nickel ferrite (CuxNi1− xFe2O4) through co-precipitation method. J. Mater. Sci. Mater. Electron 28(24), 18480–18488 (2017)
M. Mohammadikish, Hydrothermal synthesis, characterization and optical properties of ellipsoid shape α-Fe2O3 nanocrystals. Ceram. Int. 40(1), 1351–1358 (2014)
M. Singh, M. Goyal, K. Devlal, Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 12(4), 470–475 (2018). https://doi.org/10.1080/16583655.2018.1473946
M. Pudukudy, Z. Yaakob, Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J. Cluster Sci. 26(4), 1187–1201 (2015). https://doi.org/10.1007/s10876-014-0806-1
Y. Zhang et al., Photoluminescence of Fe2O3nanoparticles prepared by laser oxidation of Fe catalysts in carbon nanotubes. Mater. Res. Bull. 43(12), 3490–3494 (2008)
A. Rufus, N. Sreeju, D. Philip, Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 6(96), 94206–94217 (2016)
L. Lu, L. Li, X. Wang, G. Li, Understanding of the finite size effects on lattice vibrations and electronic transitions of nano α-Fe2O3. J. Phys. Chem. B 109(36), 17151–17156 (2005)
D.A. Wheeler, G. Wang, Y. Ling, Y. Li, J.Z. Zhang, Nanostructured hematite: synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 5(5), 6682–6702 (2012)
B.S. Zou, V. Volkov, Surface modification on time-resolved fluorescences of Fe2O3 nanocrystals. J. Phys. Chem. Solids 61(5), 757–764 (2000)
M.E. Sadat et al., Photoluminescence and photothermal effect of Fe3O4 nanoparticles for medical imaging and therapy. Appl. Phys. Lett. 105(9), 91903 (2014)
R.A. Bepari, P. Bharali, B.K. Das, J. Saudi Chem. Soc. 21, S170–S178 (2017)
M. Tadic et al., Synthesis, morphology and microstructure of pomegranate-like hematite (α-Fe2O3) superstructure with high coercivity. J. Alloy. Compd. 543, 118–124 (2012)
M. Ounacer et al., Structural and magnetic studies of annealed iron oxide nanoparticles. J. Supercond. Novel Magn. 33(10), 3249–3261 (2020). https://doi.org/10.1007/s10948-020-05586-z
N. Amin, S. Arajs, Morin temperature of annealed submicronic α-Fe2O3 particles. Phys. Rev. B 35(10), 4810 (1987)
A. Bhattacharjee, A. Rooj, M. Roy, J. Kusz, P. Gütlich, Solventless synthesis of hematite nanoparticles using ferrocene. J. Mater. Sci. 48(7), 2961–2968 (2013). https://doi.org/10.1007/s10853-012-7067-x
J. Jacob, A. Khadar, VSM and Mossbauer study of nanostructured hematite. J. Magn. Magn. Mater. 322(6), 614–621 (2010). https://doi.org/10.1016/j.jmmm.2009.10.025
S. Ghosh et al., Effect of size fractionation on purity, thermal stability and electrical properties of natural hematite. J. Electron. Mater. 50, 3836–3845 (2021)
R. Bhat, M. Qayoom, G.N. Dar, B. Want, Structural, dielectric, optical and magnetic studies of dysprosium doped iron oxide nanostructures. Mater. Chem. Phys. 245, 122764 (2020). https://doi.org/10.1016/j.matchemphys.2020.122764
M.M. Gomaa, Electrical properties of hematite and pure sand synthetic homogeneous mixture. Appl. Water Sci. 13(2), 43 (2023)
K.W. Wagner, Zur theorie der unvollkommenen dielektrika. Ann. Phys. 345(5), 817–855 (1913)
J.C. Maxwell, Electricity and Magnetism, vol. 2 (Oxford University Press, New York, 1973)
C.G. Koops, On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 83(1), 121 (1951)
S. Roy et al., Crystallinity mediated variation in optical and electrical properties of hydrothermally synthesized boehmite (γ-AlOOH) nanoparticles. J. Alloy. Compd. 763, 749–758 (2018)
R. Bhat, M. Qayoom, G.N. Dar, B. Want, Improved dielectric, conductivity and magnetic properties of erbium doped α-Fe2O3 nanoparticles. J. Mater. Sci. Mater. Electron 30(24), 20914–20934 (2019)
J.C. Papaioannou, G.S. Patermarakis, H.S. Karayianni, Electron hop** mechanism in hematite (α-Fe2O3). J. Phys. Chem. Solids 66(5), 839–844 (2005). https://doi.org/10.1016/j.jpcs.2004.11.002
M. Mumtaz, M. Rajpoot, M. Ali, S. Hussain, M. Khan, Influence of Graphene Oxide on AC-conduction of Hematite Nanoparticles. Mater. Innovations 02, 26–35 (2022). https://doi.org/10.54738/MI.2022.2103
S. Anand, V.M. Vinosel, M.A. Jenifer, S. Pauline, Dielectric Properties, Ac Electrical Conductivity and Electric Modulus Profiles of Hematite (α-Fe2O3) Nanoparticles. Int. Res. J. Eng. Technol. 4, 358 (2017)
H. Mansour et al., Structural, optical, magnetic and electrical properties of hematite (α-Fe2O3) nanoparticles synthesized by two methods: polyol and precipitation. Appl. Phys. A 123(12), 787 (2017). https://doi.org/10.1007/s00339-017-1408-1
R. Karmakar, A.K. Das, S. Pramanik, P.K. Kuiri, A.K. Meikap, Tunable dielectric properties and magneto-dielectric coupling of hematite based trap free flexible semiconductor. J. Alloy. Compd. 881, 160516 (2021)
M. Qayoom, K.A. Shah, A.H. Pandit, A. Firdous, G.N. Dar, Dielectric and electrical studies on iron oxide (α-Fe2O3) nanoparticles synthesized by modified solution combustion reaction for microwave applications. J. Electroceram. 45(1), 7–14 (2020)
N.B. Gatchakayala, R.S.R. Dachuru, Synthesis, magnetic, AC conductivity and dielectric properties of hematite nanocrystallites. Phys. Chem. Solid State 24(2), 244–248 (2023)
V. Sangeetha, K. Gayathri, P. Krishnan, N. Sivakumar, N. Kanagathara, G. Anbalagan, Growth, optical, thermal, dielectric and microhardness characterizations of melaminium bis (trifluoroacetate) trihydrate single crystal. J. Cryst. Growth 389, 30–38 (2014)
R.L. de S. e Silva, P. Banerjee, A.F. Júnior, Functional properties of donor-and acceptor-co-doped high dielectric constant zinc oxide ceramics. Phys. Chem. Chem. Phys. 21(18), 9456–9464 (2019)
A. Ghosh, Frequency-dependent conductivity in bismuth-vanadate glassy semiconductors. Phys. Rev. B 41(3), 1479 (1990)
M. Iacob et al., Iron oxide nanoparticles as dielectric and piezoelectric enhancers for silicone elastomers. Smart Mater. Struct. 26(10), 105046 (2017)
Q. Chi et al., Enhanced thermal conductivity and dielectric properties of iron oxide/polyethylene nanocomposites induced by a magnetic field. Sci. Rep. 7(1), 1–11 (2017)
Acknowledgements
Author SK is thankful to DST-INSPIRE, Government of India for providing a fellowship. This work was partially carried out using the facilities of UGC-DAE CSR, Indore. The authors acknowledge the financial support from UGC-DAE CSR through a Collaborative Research Scheme (CRS) project number CRS/2022-23/01/731. Financial support for the thermogravimetry analyzer (STA 449 F3 Jupiter) to the Department of Science and Technology, Government of India, through a grant to the Department of Physics, Visva-Bharati is gratefully acknowledged. Authors thank DST-PURSE Facility, Visva-Bharati University for providing the FESEM and EDX facilities.
Author information
Authors and Affiliations
Contributions
Material preparation, major data collection and analysis were performed by TS, while SK, GG, PKS and VRR were involved in some data collections. AB conceptualized the problem and designed the study. The first draft of the manuscript was written by TS and finalized by AB. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Authors declare that they have no conflict interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkar, T., Kundu, S., Ghorai, G. et al. Structure, optical, magnetic, morphology and dielectric studies of pristine and green synthesized hematite nanoparticles. Appl. Phys. A 130, 123 (2024). https://doi.org/10.1007/s00339-023-07228-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07228-2