Abstract
Objectives
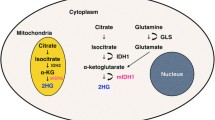
The 2021 World Health Organization (WHO) classification of central nervous system (CNS) tumors prioritizes isocitrate dehydrogenase (IDH) mutation to define tumor types in diffuse gliomas, in contrast to the 2016 classification, which prioritized histological features. Our objective was to investigate the influence of this change in the performance of proton MR spectroscopy (1H-MRS) in segregating high-grade diffuse astrocytoma subgroups.
Methods
Patients with CNS WHO grade 3 and 4 diffuse astrocytoma, known IDH mutation status, and available 1H-MRS were retrospectively retrieved and divided into 4 groups based on IDH mutation status and histological grade. Differences in 1H-MRS between groups were analyzed with the Kruskal–Wallis test. The points on the spectrum that showed the greatest differences were chosen to evaluate the performance of 1H-MRS in discriminating between grades 3 and 4 tumors (WHO 2016 defined), and between IDH-mutant and IDH-wildtype tumors (WHO 2021). ROC curves were constructed with these points, and AUC values were calculated and compared.
Results
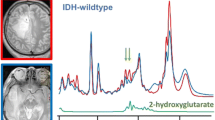
The study included 223 patients with high-grade diffuse astrocytoma. Discrimination between IDH-mutant and IDH-wildtype tumors showed higher AUC values (highest AUC short TE, 0.943; long TE, 0.864) and more noticeable visual differences than the discrimination between grade 3 and 4 tumors (short TE, 0.885; long TE, 0.838).
Conclusion
Our findings suggest that 1H-MRS is more applicable to classify high-grade astrocytomas defined with the 2021 criteria. Improved metabolomic robustness and more homogeneous groups yielded better tumor type discrimination by 1H-MRS with the new criteria.
Clinical relevance statement
The 2021 World Health Organization classification of brain tumors empowers molecular criteria to improve tumor characterization. This derives in greater segregation of high-grade diffuse astrocytoma subgroups by MR spectroscopy and warrants further development of brain tumor classification tools with spectroscopy.
Key Points
• The new 2021 updated World Health Organization classification of central nervous system tumors maximizes the role of molecular diagnosis in the classification of brain tumors.
• Proton MR spectroscopy performs better to segregate high-grade astrocytoma subgroups when defined with the new criteria.
• The study provides additional evidence of improved metabolic characterization of brain tumor subgroups with the new criteria.
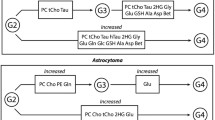
Graphical abstract





Similar content being viewed by others
Abbreviations
- 1H-MRS:
-
Proton MR spectroscopy
- CNS:
-
Central nervous system
- IDH:
-
Isocitrate dehydrogenase
- WHO:
-
World Health Organization
References
WHO Classification of Tumours Editorial Board (2021) World Health Organization classification of tumours of the central nervous system, 5th edn. International Agency for Research on Cancer, Lyon
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251
Tate AR, Underwood J, Acosta D et al (2006) Development of a decision support system for diagnosis and grading of brain tumours using in vivo magnetic resonance single voxel spectra. NMR Biomed 19:411–434
Bulik M, Jancalek R, Vanicek J et al (2013) Potential of MR spectroscopy for assessment of glioma grading. Clin Neurol Neurosurg 115:146–153
Wang Q, Zhang H, Zhang J et al (2016) The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from low-grade gliomas: a systematic review and meta-analysis. Eur Radiol 16(26):2670–2684
Castillo M, Smith JK, Kwock L (2000) Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol 21:1645–1649
Shimizu H, Kumabe T, Shirane R et al (2000) Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. AJNR Am J Neuroradiol 21:659–665
Stefan D, Cesare FD, Andrasescu A et al (2009) Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol. https://doi.org/10.1088/0957-0233/20/10/104035
Majós C, Bruna J, Julià-Sapé M et al (2011) Proton MR spectroscopy provides relevant prognostic information in high-grade astrocytomas. AJNR Am J Neuroradiol 32:74–80
Majós C, Alonso J, Aguilera C et al (2003) Proton magnetic resonance spectroscopy ((1)H MRS) of human brain tumours: assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol 13:582–591
García-Gómez JM, Luts J, Julià-Sapé M et al (2009) Multiproject-multicenter evaluation of automatic brain tumor classification by magnetic resonance spectroscopy. MAGMA 22:5–18. https://doi.org/10.1007/s10334-008-0146-y
Kim JH, Chang KH, Na DG et al (2006) 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am J Neuroradiol 27:1412–1418
Julià-Sapé M, Coronel I, Majós C et al (2012) Prospective diagnostic performance evaluation of single-voxel 1H MRS for ty** and grading of brain tumours. NMR Biomed 25:661–673
Majós C, Julià-Sapé M, Alonso J et al (2004) Brain tumor classification by proton MR spectroscopy: comparison of diagnostic accuracy at short and long TE. AJNR Am J Neuroradiol 25:1696–1704
Franco P, Huebschle I, Simon-Gabriel CP et al (2021) Map** of metabolic heterogeneity of glioma using MR-spectroscopy. Cancers (Basel) 13:2417
Osborn AG, Louis DN, Poussaint TY et al (2022) The 2021 World Health Organization classification of tumors of the central nervous system: what neuroradiologists need to know. AJNR Am J Neuroradiol 43:928–937
Suh CH, Kim HS, Jung SC et al (2018) 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol 20:1573–1583
Acknowledgements
Co-funded by European Regional Development Fund. ERDF, a way to build Europe. We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Funding
This work has received funding by the Spanish Ministry of Health, Instituto de Salud Carlos III (ISCIII) (PI20/00360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Carles Majós.
Conflict of interest
Albert Pons-Escoda is a member of the European Radiology Editorial Board. They have not taken part in the review or selection process of this article.
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in:
1. Majós C, Bruna J, Julià-Sapé M, Cos M, Camins A, Gil M, Acebes JJ, Aguilera C, Arús C (2011) Proton magnetic resonance spectroscopy provides relevant prognostic information in high-grade astrocytomas. AJNR Am J Neuroradiol 32:74–80.
2. Julià-Sapé M, Coronel I, Majós C, Candiota AP, Serrallonga M, Cos M, Aguilera C, Acebes JJ, Griffiths JR, Arús C (2012) Prospective diagnostic performance evaluation of SV-1H-MRS for ty** and grading brain tumours. NMR Biomed 25:661–673.
3. Julià-Sapé M, Majós C, Camins A, Samitier A, Baquero M, Serrallonga M, Domènech S, Grivé E, Howe F, Opstad K, Calvar J, Aguilera C, Arús C (2014) Multicentre evaluation of the INTERPRET decisión-support system 2.0 for brain tumour classification. NMR Biomed 27:1009–1028.
4. Mora P, Majós C, Castañer S, Sánchez JJ, Gabarrós A, Muntané A, Aguilera C, Arús C (2014) 1H-MRS can be used to reinforce the suspicion of primary central nervous system lymphoma prior to surgery. Eur Radiol 24:2895–2905.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carles Majós and Albert Pons-Escoda have contributed equally and are co-first authors of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majós, C., Pons-Escoda, A., Naval, P. et al. Proton MR spectroscopy shows improved performance to segregate high-grade astrocytoma subgroups when defined with the new 2021 World Health Organization classification of central nervous system tumors. Eur Radiol 34, 2174–2182 (2024). https://doi.org/10.1007/s00330-023-10138-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10138-9