Abstract
Objectives
We aimed to develop and validate a deep learning system (DLS) by using an auxiliary section that extracts and outputs specific ultrasound diagnostic features to improve the explainable, clinical relevant utility of using DLS for detecting NAFLD.
Methods
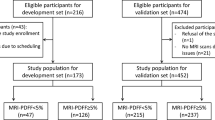
In a community-based study of 4144 participants with abdominal ultrasound scan in Hangzhou, China, we sampled 928 (617 [66.5%] females, mean age: 56 years ± 13 [standard deviation]) participants (2 images per participant) to develop and validate DLS, a two-section neural network (2S-NNet). Radiologists’ consensus diagnosis classified hepatic steatosis as none steatosis, mild, moderate, and severe. We also explored the NAFLD detection performance of six one-section neural network models and five fatty liver indices on our data set. We further evaluated the influence of participants’ characteristics on the correctness of 2S-NNet by logistic regression.
Results
Area under the curve (AUROC) of 2S-NNet for hepatic steatosis was 0.90 for ≥ mild, 0.85 for ≥ moderate, and 0.93 for severe steatosis, and was 0.90 for NAFLD presence, 0.84 for moderate to severe NAFLD, and 0.93 for severe NAFLD. The AUROC of NAFLD severity was 0.88 for 2S-NNet, and 0.79–0.86 for one-section models. The AUROC of NAFLD presence was 0.90 for 2S-NNet, and 0.54–0.82 for fatty liver indices. Age, sex, body mass index, diabetes, fibrosis-4 index, android fat ratio, and skeletal muscle via dual-energy X-ray absorptiometry had no significant impact on the correctness of 2S-NNet (p > 0.05).
Conclusions
By using two-section design, 2S-NNet had improved the performance for detecting NAFLD with more explainable, clinical relevant utility than using one-section design.
Key Points
• Based on the consensus review derived from radiologists, our DLS (2S-NNet) had an AUROC of 0.88 by using two-section design and yielded better performance for detecting NAFLD than using one-section design with more explainable, clinical relevant utility.
• The 2S-NNet outperformed five fatty liver indices with the highest AUROCs (0.84–0.93 vs. 0.54–0.82) for different NAFLD severity screening, indicating screening utility of deep learning-based radiology may perform better than blood biomarker panels in epidemiology.
• The correctness of 2S-NNet was not significantly influenced by individual's characteristics, including age, sex, body mass index, diabetes, fibrosis-4 index, android fat ratio, and skeletal muscle via dual-energy X-ray absorptiometry.



Similar content being viewed by others
Abbreviations
- AUROC:
-
Area under the receiver operating characteristic curve
- BL:
-
“Bright liver”
- DLS:
-
Deep learning system
- DXA:
-
Dual-energy X-ray absorptiometry
- IDB:
-
Intrahepatic ducts blurring
- IVD:
-
Impaired visualization of more than a half of the diaphragm
- LMICs:
-
Low-middle-income countries
- NAFLD:
-
Nonalcoholic fatty liver disease
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
References
Le MH, Yeo YH, Li X et al (2021) 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol S1542–3565(21):01280–01285
Wu Y, Zheng Q, Zou B et al (2020) The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int 14:259–269
Zhou F, Zhou J, Wang W et al (2019) Unexpected rapid increase in the burden of NAFLD in China From 2008 to 2018: a systematic review and meta-analysis. Hepatology 70:1119–1133
Wang XJ, Malhi H (2018) Nonalcoholic fatty liver disease. Ann Intern Med 169(9):ITC65–ITC80
Fan JG, Wei L, Zhuang H et al (2019) Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis 20(4):163–173
Hardy T, Oakley F, Anstee QM, Day CP (2016) Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol 11:451–496
Huang DQ, El-Serag HB, Loomba R (2021) Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 18:223–238
Rinella ME (2015) Nonalcoholic fatty liver disease: a systematic review. JAMA 313:2263–2273
Brunner KT, Henneberg CJ, Wilechansky RM, Long MT (2019) Nonalcoholic fatty liver disease and obesity treatment. Curr Obes Rep 8:220–228
Starekova J, Hernando D, Pickhardt PJ, Reeder SB (2021) Quantification of liver fat content with CT and MRI: state of the art. Radiology 301:250–262
Ferraioli G, Soares Monteiro LB (2019) Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol 25:6053–6062
Byra MMB, Styczynski G, G, C Szmigielski C, et al (2018) Transfer learning with deep convolutional neural network for liver steatosis assessment in ultrasound images. Int J Comput Assist Radiol Surg 13(12):1895–1903
Biswas M, Kuppili V, Edla DR et al (2018) Symtosis: a liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. Comput Methods Programs Biomed 155:165–177
Han A, Byra M, Heba E et al (2020) Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat with radiofrequency ultrasound data using one-dimensional convolutional neural networks. Radiology 295:342–350
Kim T, Lee DH, Park E-K, Choi S (2021) Deep learning techniques for fatty liver using multi-view ultrasound images scanned by different scanners: development and validation study. JMIR Med Inform 9:e30066
Che H, Brown LG, Foran DJ, Nosher JL, Hacihaliloglu I (2021) Liver disease classification from ultrasound using multi-scale CNN. Int J Comput Assist Radiol Surg 16:1537–1548
Zamanian H, Mostaar A, Azadeh P, Ahmadi M (2021) Implementation of combinational deep learning algorithm for non-alcoholic fatty liver classification in ultrasound images. J Biomed Phys Eng 11:73–84
Chen J-R, Chao Y-P, Tsai Y-W et al (2020) Clinical value of information entropy compared with deep learning for ultrasound grading of hepatic steatosis. Entropy (Basel) 22:E1006
Sanabria SJ, Pirmoazen AM, Dahl J, Kamaya A, El Kaffas A (2022) Comparative study of raw ultrasound data representations in deep learning to classify hepatic steatosis. Ultrasound Med Biol 48:2060–2078
Byra M, Han A, Boehringer AS et al (2022) Liver fat assessment in multiview sonography using transfer learning with convolutional neural networks. J Ultrasound Med 41:175–184
Cha DI, Kang TW, Min JH et al (2021) Deep learning-based automated quantification of the hepatorenal index for evaluation of fatty liver by ultrasonography. Ultrasonography 40:565–574
Troelstra MA, Van Dijk A-M, Witjes JJ et al (2022) Self-supervised neural network improves tri-exponential intravoxel incoherent motion model fitting compared to least-squares fitting in non-alcoholic fatty liver disease. Front Physiol 13:942495
Constantinescu EC, Udriștoiu A-L, UdriștoiuȘtefan C et al (2021) Transfer learning with pre-trained deep convolutional neural networks for the automatic assessment of liver steatosis in ultrasound images. Med Ultrason 23:135–139
Li B, Tai D-I, Yan K et al (2022) Accurate and generalizable quantitative scoring of liver steatosis from ultrasound images via scalable deep learning. World J Gastroenterol 28:2494–2508
Rhyou S-Y, Yoo J-C (2021) Cascaded deep learning neural network for automated liver steatosis diagnosis using ultrasound images. Sensors (Basel) 21:5304
Chou T-H, Yeh H-J, Chang C-C et al (2021) Deep learning for abdominal ultrasound: a computer-aided diagnostic system for the severity of fatty liver. J Chin Med Assoc 84:842–850
Ci C, Tb C, Nh L et al (2019) Classification for liver ultrasound tomography by posterior attenuation correction with a phantom study. Proc Inst Mech Eng H 233(11):1100–1112
Cao W, An X, Cong L, Lyu C, Zhou Q, Guo R (2020) Application of deep learning in quantitative analysis of 2-dimensional ultrasound imaging of nonalcoholic fatty liver disease. J Ultrasound Med 39(1):51–59
Saba L, Dey N, Ashour AS et al (2016) Automated stratification of liver disease in ultrasound: an online accurate feature classification paradigm. Comput Methods Programs Biomed 130:118–134
Reddy DS, Bharath R, Rajalakshmi P (2018) A novel computer-aided diagnosis framework using deep learning for classification of fatty liver disease in ultrasound imaging. 2018 IEEE 20th international conference on e-health networking, applications and services (Healthcom). IEEE, 2018:1–5
Zhang P, Ge Z, Wang H et al (2018) Prolactin improves hepatic steatosis via CD36 pathway. J Hepatol 68:1247–1255
Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ (2009) Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 51:1061–1067
Min Y, Zhao X, Stafford RS et al (2021) Cohort profile: WELL Living Laboratory in China (WELL-China). Int J Epidemiol 50(5):1432–1443
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition. 2016:770–778
Han D, Kim J, Kim J (2017) Deep pyramidal residual networks. Proceedings of the IEEE conference on computer vision and pattern recognition. 2017:5927–5935
Tan M, Le QV (2020) EfficientNet: rethinking model scaling for convolutional neural networks. International conference on machine learning. PMLR, 2019:6105–6114
Sandler M, Howard A, Zhu M, et al (2019) MobileNetV2: inverted residuals and linear bottlenecks. Proceedings of the IEEE conference on computer vision and pattern recognition. 2018:4510–4520
Bedogni G, Bellentani S, Miglioli L et al (2006) The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6:33
Lee J-H, Kim D, Kim HJ et al (2010) Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 42:503–508
Li L, You W, Ren W (2017) The ZJU index is a powerful index for identifying NAFLD in the general Chinese population. Acta Diabetol 54:905–911
Yip TC-F, Ma AJ, Wong VW-S et al (2017) Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population. Aliment Pharmacol Ther 46:447–456
Zhou Y-J, Zhou Y-F, Zheng J-N et al (2017) NAFL screening score: a basic score identifying ultrasound-diagnosed non-alcoholic fatty liver. Clin Chim Acta 475:44–50
Kong X, Ai B, Kong Y et al (2019) Artificial intelligence: a key to relieve China’s insufficient and unequally-distributed medical resources. Am J Transl Res 11:2632–2640
Byrne CD, Targher G (2016) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: is universal screening appropriate? Diabetologia 59:1141–1144
Subramanya MB, Kumar V, Mukherjee S, Saini M (2015) A CAD system for B-mode fatty liver ultrasound images using texture features. J Med Eng Technol 39(2):123–130
Li G, Luo Y, Deng W, Xu X, Liu A, Song E (2008) Computer aided diagnosis of fatty liver ultrasonic images based on support vector machine. Annu Int Conf IEEE Eng Med Biol Soc 2008:4768–4771
Chen J-R, Chao Y-P, Tsai Y-W et al (2020) Clinical value of information entropy compared with deep learning for ultrasound grading of hepatic steatosis. Entropy (Basel) 22(9):1006
Acknowledgements
Initial foundational funding for the Stanford Wellness Living laboratory (WELL) was provided by Amway via an unrestricted gift through the Nutrilite Health Institute Wellness Fund to Stanford University. Through Zhejiang University, the Cyrus Tang Foundation, Hsun K Chou Fund, and Zhejiang University Education Foundation also provided important financial support for the study. Ying Lu kindly provided statistical advice for this manuscript.
Funding
Initial foundational funding for the Stanford Wellness Living laboratory (WELL) was provided by Amway via an unrestricted gift through the Nutrilite Health Institute Wellness Fund to Stanford University. Through Zhejiang University, the Cyrus Tang Foundation, Hsun K Chou Fund, and Zhejiang University Education Foundation also provided important financial support for the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shankuan Zhu (one of the corresponding authors).
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Ying Lu kindly provided statistical advice for this manuscript, and he is one of the authors.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.The study has obtained the Institution Review Board approvals from both Zhejiang University (No. ZGL201507-3) and Stanford University (IRB-35020).
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Yang and **g Liu contributed equally as first authors.
Ann W. Hsing, Jian Wu, and Shankuan Zhu contributed equally as senior authors.
Summary statement: By using two-section design, our newly developed deep learning system (2S-NNet) had high accuracies and AUROCs for hepatic steatosis and NAFLD detection based on radiologists’ diagnosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Liu, J., Sun, C. et al. Nonalcoholic fatty liver disease (NAFLD) detection and deep learning in a Chinese community-based population. Eur Radiol 33, 5894–5906 (2023). https://doi.org/10.1007/s00330-023-09515-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09515-1