Abstract
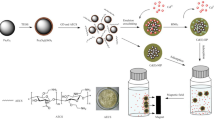
In this research copper tagged magnetic surface imprinted polymer (IIP) has been developed using 1,10-phenanthroline immobilized magnesium ferrite–starch. Maximum removal of 95% was achieved at the optimum conditions i.e., 10 min contact time, pH of 6, and IIP amount of 20 mg. According to the kinetic study copper adsorption followed pseudo-second-order model moreover, based on the isotherm study Freundlich model better described the adsorption process as the maximum adsorption capacity of 33.7 mg g−1 was achieved. Adsorbed copper ions were eluted from the adsorbent surface with an HCl solution (0.5 mol L−1) and the IIP exhibited appropriate reusability (removal of more than 92%) after four cycles of sorption and elution. The limit of detection, linear dynamic range and RSD were 0.5 and 3.0–100 µg L−1 and 3%, respectively. The copper-tagged IIP showed a selectivity factor of 8.8, 4.6, 3.3, 3.2 and 5.4 concerning Zn, Ni, Co, Mn and Cd, respectively. The developed IIP-based adsorption system was employed for copper adsorption/preconcentration from water and food samples with an efficiency more than of 95%. Results confirmed that the IIP system is an appropriate route to reduce the side effects of heavy metals in aqueous media.







Similar content being viewed by others
References
Nchoe OB, Klink MJ, Mtunzi FM, Pakade VE (2020) Synthesis, characterization, and application of β-cyclodextrin-based ion-imprinted polymer for selective sequestration of Cr(VI) ions from aqueous media: Kinetics and isotherm studies. J Mol Liq 298:111991. https://doi.org/10.1016/j.molliq.2019.111991
Hassan Amini M, Alijani H, Hossein Beyki M (2022) Toxic cadmium selective sequestration from food samples using melamine anchored magnetic cellulose by surface imprinting route. Food Chem 396:133688. https://doi.org/10.1016/j.foodchem.2022.133688
Silva Filho EC, Lima LCB, Silva FC et al (2013) Immobilization of ethylene sulfide in aminated cellulose for removal of the divalent cations. Carbohydr Polym 92:1203–1210. https://doi.org/10.1016/j.carbpol.2012.10.031
Zhang X, Wang H, Sun X et al (2019) Preparation and properties of thermo-sensitive surface Pb(II) ion-imprinted polymers. Colloids Surfaces A 577:138–146. https://doi.org/10.1016/j.colsurfa.2019.05.064
Roushani M, Abbasi S (2015) Synthesis and application of ion-imprinted polymer nanoparticles for the extraction and preconcentration of copper ions in environmental water samples. Environ Monit Assess 187:219. https://doi.org/10.1007/s10661-015-4468-8
Kuras MJ, Wie E (2015) Synthesis and characterization of a new copper(II) ion-imprinted polymer. Polym Bull 72:3227–3240. https://doi.org/10.1007/s00289-015-1463-8
Yilmaz V, Arslan Z, Hazer O, Yilmaz H (2014) Selective solid phase extraction of copper using a new Cu(II) -imprinted polymer and determination by inductively coupled plasma optical emission spectroscopy ( ICP-OES ). Microchem J 114:65–72. https://doi.org/10.1016/j.microc.2013.12.002
Beyki MH, Shemirani F, Khani R (2014) Green preconcentration of trace amounts of copper from water and food samples onto novel organo-nanoclay prior to flame atomic absorption spectrometry. J AOAC Int 97:1426–1433. https://doi.org/10.5740/jaoacint.12-212
Taylor P, Zeng J, Chen H et al (2014) A ion-imprinted chitosan/Al2O3 composite material for selective separation of copper(II). Desalin Water Treat. https://doi.org/10.1080/19443994.2014.923332
Kong D, Qiao N, Liu H et al (2017) Fast and efficient removal of copper using sandwich-like graphene oxide com- posite imprinted materials. Chem Eng J 326:141–150. https://doi.org/10.1016/j.cej.2017.05.140
Nasab SG, Semnani A, Karimi M (2019) Synthesis of ion-imprinted polymer-decorated SBA-15 as a selective and efficient system for the removal and extraction of Cu(II) with focus on optimization by response surface methodology. Analyst 144:4596–4612. https://doi.org/10.1039/c9an00586b
Shahrashoub M, Bakhtiari S (2021) The efficiency of activated carbon/ iron oxide nanoparticles composites in copper removal: industrial waste recovery, green synthesis, characterization, and adsorption—desorption studies. Microporous Mesoporous Mater 311:110692. https://doi.org/10.1016/j.micromeso.2020.110692
Kong L, Liu X, Lv G et al (2022) Copper adsorption using hydroxyapatite derived from bovine bone. Adv Civ Eng 2022:1–10
Buema G, Trifas L, Harja M (2021) Removal of Toxic copper ion from aqueous media by adsorption on fly ash-derived zeolites: kinetic and equilibrium studies. Polymers (Basel) 13:3468
El AMR, Hassan HS, Elkady MF, Elzain MSMAA (2019) Isothermal, kinetic, and thermodynamic studies on copper adsorption on modified styrene acrylonitrile copolymer. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-02199-x
Rais S, Islam A, Ahmad I et al (2021) Preparation of a new magnetic ion-imprinted polymer and optimization using Box-Behnken design for selective removal and determination of Cu(II) in food and wastewater samples. Food Chem 334:127563. https://doi.org/10.1016/j.foodchem.2020.127563
Khajeh M, Heidari ZS, Sanchooli E (2011) Synthesis, characterization and removal of lead from water samples using lead-ion imprinted polymer. Chem Eng J 166:1158–1163. https://doi.org/10.1016/j.cej.2010.12.018
Kong D, Wang N, Qiao N et al (2017) Facile preparation of ion-imprinted chitosan microspheres enwrap** Fe3O4 and graphene oxide by inverse suspension cross-linking for highly selective removal of Copper(II). ACS Sustain Chem Eng 5:7401–7409. https://doi.org/10.1021/acssuschemeng.7b01761
Liang J, Liu J, Yuan X et al (2015) Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chem Eng J 273:101–110. https://doi.org/10.1016/j.cej.2015.03.069
Zhang W, Yun M, Yu Z et al (2019) A novel Cu(II) Ion—imprinted alginate–chitosan complex adsorbent for selective separation of Cu(II) from aqueous. Polym Bull 76:1861–1876. https://doi.org/10.1007/s00289-018-2433-8
Jagirani MS, Balouch A, Alveroğlu E, Mahesar SA, Zeytuncu B, A&ARK (2022) Fabrication of cobalt tagged smart ion-imprinted polymeric material applied for the elimination of Co2+ ions from real environmental samples. Polym Bull 79:10135–10153
Shamsipur M, Besharati-seidani A, Fasihi J, Sharghi H (2010) Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper(II) ions. Talanta 83:674–681. https://doi.org/10.1016/j.talanta.2010.10.021
Photopolymerization R, Msaadi R, Yilmaz G et al (2019) Highly selective copper ion imprinted clay/polymer nanocomposites prepared by visible light initiated. Polymers (Basel) 11:286. https://doi.org/10.3390/polym11020286
Shamsipur M, Besharati-seidani A (2011) Synthesis of a novel nanostructured ion-imprinted polymer for very fast and highly selective recognition of copper(II) ions in aqueous media. React Funct Polym 71:131–139. https://doi.org/10.1016/j.reactfunctpolym.2010.11.002
Ebrahimzadeh H, Behbahani M, Yamini Y, Adlnasab L (2013) Optimization of Cu(II)-ion imprinted nanoparticles for trace monitoring of copper in water and fish samples using a Box–Behnken design. React Funct Polym 73:23–29. https://doi.org/10.1016/j.reactfunctpolym.2012.10.006
Jiang Y, Tang B, Zhao P et al (2021) Synthesis of copper and lead ion imprinted polymer submicron spheres to remove—Cu2+ and—Pb2+. J Inorg Organomet Polym Mater 31:4628–4636. https://doi.org/10.1007/s10904-021-02065-3
Hou L, Yang C, Rao X et al (2021) Fabrication of recoverable magnetic surface ion-imprinted polymer based on graphene oxide for fast and selective removal of lead ions from aqueous solution. Colloids Surfaces A Physicochem Eng Asp 625:126949. https://doi.org/10.1016/j.colsurfa.2021.126949
Dong C, Shi H, Han Y et al (2021) Molecularly imprinted polymers by the surface imprinting technique. Eur Polym J 145:110231. https://doi.org/10.1016/j.eurpolymj.2020.110231
Fayazi M, Ali M, Afzali D et al (2016) Synthesis and application of novel ion-imprinted polymer coated magnetic multi-walled carbon nanotubes for selective solid phase extraction of lead(II) ions. Mater Sci Eng C 60:365–373. https://doi.org/10.1016/j.msec.2015.11.060
Ghanei-motlagh M, Taher MA (2017) Magnetic silver (I) ion-imprinted polymeric nanoparticles on a carbon paste electrode for voltammetric determination of silver (I). Microchim Acta 184:1691–1699. https://doi.org/10.1007/s00604-017-2157-8
Fayazi M, Taher MA, Salavati MR (2016) Synthesis and application of a novel nanostructured ion-imprinted polymer for the preconcentration and determination of thallium (I) ions in water samples. J Hazard Mater 309:27–36. https://doi.org/10.1016/j.jhazmat.2016.02.002
Ma X, Liu X, Anderson DP, Chang PR (2015) Modification of porous starch for the adsorption of heavy metal ions from aqueous solution. Food Chem 181:133–139. https://doi.org/10.1016/j.foodchem.2015.02.089
Baykal G, Kazan D, Seval K, Duygu Takanoglu Bulut SG, Elci UD, Akdogan A (2021) Determination of lead and cadmium in water samples by magnetic solid-phase extraction with iron oxide@silicon oxide-graphene oxide (Fe3O4@SiO2-GO) hybrid magnetic nanoparticles and microinjection sampling flame atomic absorption spectrometry. Instrum Sci Technol 49:258–275
Guo S, Zhang F, Li D, Jiao P (2020) Highly efficient and selective removal of cadmium from aqueous solutions based on magnetic graphitic carbon nitride materials with molecularly imprinted polymers. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.128887
Rezaei AA, Hossein Beyki M, Shemirani F (2017) Fast sono assisted ferrofluid mediated silver super—adsorption over magnesium ferrite–copper sulfide chalcogenide with the aid of multivariate optimization. Ultrason Sonochem 37:509–517. https://doi.org/10.1016/j.ultsonch.2017.02.002
Mohammad Hassan Amini MHB (2023) Constraction of 1,10-phenanthroline functionalized magnetic starch as a lead(II) tagged surface imprinted biopolymer for highly selective targeting of toxic lead ions. Int J Biol Macromol 242:124996. https://doi.org/10.1016/j.ijbiomac.2023.124996
Beyki Shemirani FMH (2015) Dual application of facilely synthesized Fe3O4 nanoparticles: fast reduction of nitro compound and preparation of magnetic polyphenylthiourea nanocomposite for efficient adsorption of lead ions. RSC Adv 5:22224–22233. https://doi.org/10.1039/c4ra12549e
Yusof NF, Mehamod FS, Mohd Suah FB (2019) Fabrication and binding characterization of ion imprinted polymers for highly selective Co2+ ions in an aqueous medium. J Environ Chem Eng 7:103007. https://doi.org/10.1016/j.jece.2019.103007
Li J, Yuan H, Li G et al (2010) Cation distribution dependence of magnetic properties of solgel prepared MnFe2O4 spinel ferrite nanoparticles. J Magn Magn Mater 322:3396–3400. https://doi.org/10.1016/j.jmmm.2010.06.035
Khafagy RM (2011) Synthesis, characterization, magnetic and electrical properties of the novel conductive and magnetic polyaniline/MgFe2O4 nanocomposite having the core-shell structure. J Alloys Compd 509:9849–9857. https://doi.org/10.1016/j.jallcom.2011.07.008
Mostafa Hossein Beyki FS (2017) Magnetic ZnFe2O4 @polyhydroxybenzoic acid nanostructure for efficient B. subtilis capturing. Nannomedicine Res J 2:165–170
Beyki MH, Bayat M, Miri S et al (2014) Synthesis, characterization, and silver adsorption property of magnetic cellulose xanthate from acidic solution: Prepared by one step and biogenic approach. Ind Eng Chem Res 53:14904–14912. https://doi.org/10.1021/ie501989q
Hossein Beyki M, Miri S, Shemirani F et al (2016) A new derivative of core–shell magnetic chitosan biopolymer: synthesis characterization and application for adsorption of lead and copper ions. Clean: Soil, Air, Water. https://doi.org/10.1002/clen.201400244
Bayat M, Beyki MH, Shemirani F (2015) One-step and biogenic synthesis of magnetic Fe3O4-fir sawdust composite: application for selective preconcentration and determination of gold ions. J Ind Eng Chem 21:912–919. https://doi.org/10.1016/j.jiec.2014.04.032
Lv H, Zhang H, Ji G (2016) Development of novel graphene/g-C3N4 composite with broad-frequency and light-weight features. Part Part Syst Charact 33:656–663. https://doi.org/10.1002/ppsc.201600065
Wang W, Ding Z, Wu S et al (2015) Ethanol-assisted synthesis and adsorption property of flake-like NiFe2O4 nanoparticles. Ceram Int 41:13624–13629. https://doi.org/10.1016/j.ceramint.2015.07.159
Wu X, Wang W, Song N et al (2016) From nanosphere to nanorod: tuning morphology, structure and performance of cobalt ferrites via Pr3+ do**. Chem Eng J 306:382–392. https://doi.org/10.1016/j.cej.2016.07.070
Girija P, Mathew B (2021) Synthesis and characterization of metal ion imprinted alginate network. Int J Res Eng Sci 09:33–35
Saatçilar Ö, Şatiroǧlu N, Say R et al (2006) Binding behavior of Fe3+ ions on ion-imprinted polymeric beads for analytical applications. J Appl Polym Sci 101:3520–3528. https://doi.org/10.1002/app.24591
Abollino O, Aceto M, Malandrino M et al (2003) Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res 37:1619–1627
Suriyanon N, Punyapalakul P, Ngamcharussrivichai C (2013) Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem Eng J 214:208–218. https://doi.org/10.1016/j.cej.2012.10.052
Beyki Shemirani FMH (2015) Dual application of facilely synthesized Fe3O4 nanoparticles: fast reduction of nitro compound and preparation of magnetic polyphenylthiourea nanocomposite for efficient adsorption of lead ions. RSC Adv. https://doi.org/10.1039/c4ra12549e
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133:304–308. https://doi.org/10.1016/j.jhazmat.2005.10.016
de Liss F, López M, Khan S, Augusto M, Neto G et al (2021) Systematic study on the synthesis of novel ion-imprinted polymers based on rhodizonate for the highly selective removal of Pb(II). React Funct Polym 159:104805. https://doi.org/10.1016/j.reactfunctpolym.2020.104805
Acknowledgements
The financial support from the Imam Khomeini International University and Islamic Azad University Roudehen Branch, through the grant is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript Roya Kiani Anbouhi: Conceptualization, Methodology / Study design, Supervision, Writing – review and editing Nasrin Masnabadi : Validation, Data curation, Writing – review and editing, Visualization Mohammad Hadi Ghasemi: Validation, Writing – review and editing, Visualization, Investigation Mostafa Hossein Beyki: Writing – original draft, Investigation, Writing – review and editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no personal relationships and known competing financial interests that could have appeared to influence the presented research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anbouhi, R.K., Masnabadi, N., Ghasemi, M.H. et al. Cu(II) tagged magnetic MgFe2O4: starch based surface imprinted polymer for selective copper targeting from aqueous media. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05348-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05348-0