Abstract
Abstract
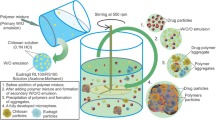
The present research aim is to develop the tablet formulation containing microspheres and encapsulating microsphere with antidiabetic drug specially Sitagliptin Phosphate Monohydrate and poly lactic co glycolic acid (PLGA) as a polymer. Preformulation study of the selected drug was carried out. Then, the microspheres were prepared by factorial design, i.e., Design-Expert 12 software (Stat-Ease Inc., USA) was used for designing of experiment, to study interaction between independent variables and dependent variables and deriving optimum formulation. Spray dryer was used for preparation of microspheres. The zeta potential, particle size, infrared spectral analysis, scanning electron microscopy, % drug content, encapsulation efficiency and % drug release were evaluated. Optimized batch F3 showed an average particle size 2500.6 nm. The in vitro drug release at 12th hour and % drug entrapment efficiency of optimized batch F3 was found to be 92.35% and the 34.00%, respectively. The final product has lowered particle size 2500.6 nm and it is focused for well acceptable and suitable due to significant drug release. The final tablet was prepared from optimized batch F3. The precompression parameters, i.e., flow properties of granules, were carried out, and then, post-compression parameters like weight variation, thickness, hardness, friability, % drug content and % drug release were evaluated. The stability study and in vitro antidiabetic study was carried out for tablet. The final product is well acceptable, suitable, palatable, easy for administration and elegant.
Graphical abstract








Similar content being viewed by others
References
Ginter E, Simko V (2012) Type 2 Diabetes mellitus-pandemic in 21st century. Adv Exp Med Biol 771: 42–50. doi: 10.1007/978-1-4614-5441-0_6
Asif AH, Sree H, Puttaswamy NH, Al-Dhubiab B (2018) An effective delivery system of sitagliptin using optimized mucoadhesive nanoparticles. Appl Sci 861:1–13
Kidambi S, Patel SB (2008) Diabetes mellitus considerations for dentistry. J Am Dent Assoc 139:8S-18S
Tahrani AA, Bailey CJ, Prato SD, Anthony HB (2011) Management of type 2 diabetes: new and future developments in treatment. The Lancet 378:182–197
Goldenberg R, Punthakee Z (2013) Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 37:S8–S11
Sree H, Attimard M, Khan TA, Nair AB, Aldhubiab BE, Sangi S, Shariff A (2013) Design and formulation of mucoadhesive microspheres of sitagliptin. J Microencapsul 30:257–264
Patel NR, Patel DA, Bharadia PD, Pandya V, Modi D (2011) Microsphere as a novel drug delivery. Int J Pharm Life Sci 2:992–997
Sanatkar R, Mohammad GRKS, Karimi E, Oskoueian E, Hendra R (2021) Evaluation of daidzein-loaded chitosan microcapsules for the colon cancer drug delivery: synthesis, characterization and release behaviour. Polym Bull. https://doi.org/10.1007/s00289-021-03853-0
Beyrami M, Karimi E, Oskoueian E (2020) Synthesized chrysin-loaded nanoliposomes improves cadmium-induced toxicity in mice. Environ Sci Pollut Res Int 27(32):40643–40651. https://doi.org/10.1007/s11356-020-10113-7
Rahmani F, Karimi E, Oskoueian E (2020) Synthesis and characterisation of chitosan-encapsulated genistein: its anti-proliferative and anti-angiogenic activities. J Microencapsul 37(4):305–313. https://doi.org/10.1080/02652048.2020.1740804
Patil P, Salunkhe V, Bhinge S, Patil S (2021) Formulation optimization and evaluation of glycyrrhetinic acid loaded PLARosome using factorial design: In-vitro anti-ulcer activity. J Indian Chem Soc. https://doi.org/10.1016/j.jics.2021.100199
Vambhurkar GB, Jagatap AM, Gavade AS, Randive DS, Bhutkar MA, Bhinge SD (2021) Formulation and evaluation 0f tablet containing pioglitazone HCL microspheres. J Rep Pharm Sci 10(1):36–41. https://doi.org/10.4103/jrptps.JRPTPS_29_19
Salunkhe VR, Patil PS, Wadkar GH, Bhinge SD (2021) Herbal liposomes: natural network for targeted drug delivery system. J Pharm Res Int 33(29B):31–41
Patil OA, Patil IS, Mane RU, Randive DS, Bhutkar MA, Bhinge SD (2018) Formulation optimization and evaluation of cefdinir nanosuspension using 23 Factorial design. Marmara Pharm J 22(4):587–598. https://doi.org/10.12991/jrp.2018.101
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8(1):102. https://doi.org/10.1186/1556-276X-8-102
Bhattacharya S (2009) Phytosomes: emerging strategy in delivery of herbal drugs and nutraceuticals. Pharma Times 41(3):9–12
Das MK, Ahmed AB, Saha D (2019) Microsphere a drug delivery system–a review. Int J Curr Pharm Res 11(4):34–41
Kumar A, Mahajan S, Bhandari N (2017) Microspheres: a review. World J Pharm Pharm Sci 6(4):724–740
Babic A, Vinet L, Chellakudam V, Janikowska K, Allémann E, Lange N (2018) Squalene-PEG-Exendin as high-affinity constructs for pancreatic beta-cell. Bioconjug Chem 29:2531–2540
Smink AM, De Haan BJ, Lakey JRT, De Vos P (2018) Polymer scaffolds for pancreatic islet transplantation-progress and challenges. Am J Transplant 18:2113–2119
Vishwakarma G, Pandey SP, Candel HS (2015) Preformulation studies of stavudine for targeted drug delivery system. Int Res J Pharm 6:640–643
Kala S, Juyal D (2016) Preformulation and characterization studies of aceclofenac active ingredient. J Pharm Innov 5:110–119
Ravisankar P, Mounika G, Devadasu C, Devala Rao G (2014) A simple validated UV Spectrophotometric method for quantitative analysis of sitagliptin phosphate in pharmaceutical dosage form. J Chem Pharm Sci 7:254–258
Stofella NCF, Veiga A, Oliveira LJ, Montin EF, Andreazza IF (2019) Solid-state characterization of different crystalline forms of sitagliptin. Mater MDPI 12:1–16
Parmar H, Bakliwal S, Gujarathi N, Rane B, Pawar S (2010) Different methods of formulation and evaluation of mucoadhesive microsphere. Int J Appl Biol Pharm 1:1157–1167
Revathi S, Dhanaraju Md (2018) Fabrication and effect of process variables of sitagliptin microspheres. Asian J Pharm Clin Res 11:291–297
Katsarov PD, Pilicheva BA, Manev HM, Lukova PK, Kassarova MI (2017) Optimization of chitosan microspheres spray drying via 32 full factorial design. Folia Med 59:310–317
Pagar PS, Savkare AD (2017) Formulation and evaluation of omeprazole microspheres by different techniques. Indo Am J Pharm Res 7:426–440
Kamble MS, Mane OR, Borwandkar VG, Mane SS, Chaudhari PD (2012) Formulation and evaluation of clindamycin HCL–chitosan microspheres for dry powder inhaler formulation. Drug Invent Today 4:527–530
Vinchurkar K, Mane S, Balekar N, Jain DK (2017) Formulation and evaluation of tablets containing artemether microspheres and lumefantrine. J Drug Deliv Ther 7:4–7
Lachman L, Herbert AL (1991) The theory and practice of industrial pharmacy. Special Indian 3rd edn, Varghese Publishing House, pp 296–317 (ISBN 0-8121-0977-5)
Malik K, Arora G, Singh I (2011) Taste masked microspheres of ofloxacin: formulation and evaluation of orodispersible tablets. Sci Pharm 79:653–672
Sangeetha R, Vedasree N (2012) In Vitro α-amylase inhibitory activity of the leaves of thespesia populnea. ISRN Pharmacology 2012:515634. https://doi.org/10.5402/2012/515634
Acknowledgements
We express our sincere thanks to Dr. C. S. Magdum, Principal, Rajarambapu College of Pharmacy, Kasegaon, MS—415404, INDIA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mulla, T.S., Salunkhe, V.R., Bhinge, S.D. et al. Formulation, design and optimization of antidiabetic drug loaded microspheres. Polym. Bull. 80, 6171–6196 (2023). https://doi.org/10.1007/s00289-022-04325-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04325-9