Abstract
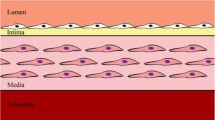
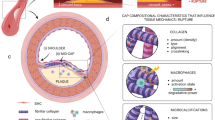
Atherosclerosis is characterised by the growth of fatty plaques in the inner artery wall. In mature plaques, vascular smooth muscle cells (SMCs) are recruited from adjacent tissue to deposit a collagenous cap over the fatty plaque core. This cap isolates the thrombogenic plaque content from the bloodstream and prevents the clotting cascade that leads to myocardial infarction or stroke. Despite the protective role of the cap, the mechanisms that regulate cap formation and maintenance are not well understood. It remains unclear why some caps become stable, while others become vulnerable to rupture. We develop a multiphase PDE model with non-standard boundary conditions to investigate collagen cap formation by SMCs in response to diffusible growth factor signals from the endothelium. Platelet-derived growth factor stimulates SMC migration, proliferation and collagen degradation, while transforming growth factor (TGF)-\(\beta \) stimulates SMC collagen synthesis and inhibits collagen degradation. The model SMCs respond haptotactically to gradients in the collagen phase and have reduced rates of migration and proliferation in dense collagenous tissue. The model, which is parameterised using in vivo and in vitro experimental data, reproduces several observations from plaque growth in mice. Numerical and analytical results demonstrate that a stable cap can be formed by a relatively small SMC population and emphasise the critical role of TGF-\(\beta \) in effective cap formation. These findings provide unique insight into the mechanisms that may lead to plaque destabilisation and rupture. This work represents an important step towards the development of a comprehensive in silico plaque model.











Similar content being viewed by others
References
Adiguzel E, Ahmad PJ, Franco C, Bendeck MP (2009) Collagens in the progression and complications of atherosclerosis. Vasc Med 14:73–89
Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS (2008) In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-\(\beta \)1. Blood 112:3650–3660
Alexander MR, Owens GK (2012) Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 74:13–40
Astanin S, Preziosi L (2008) Multiphase models of tumour growth. In: Angelis A, Chaplain MAJ, Bellomo N (eds) Selected topics in cancer modeling: genesis, evolution, immune competition, and therapy. Modeling and simulation in science, engineering and technology. Birkhäuser, Boston, pp 223–253
Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118:692–702
Bhui R, Hayenga HN (2017) An agent-based model of leukocyte transendothelial migration during atherogenesis. PLoS Comput Biol 13:e1005523
Borrelli V, di Marzo L, Sapienza P, Colasanti M, Moroni E, Cavallaro A (2006) Role of platelet-derived growth factor and transforming growth factor \(\beta _1\) in the regulation of metalloproteinase expressions. Surgery 140:454–463
Breton M, Berrou E, Brahimi-Horn MC, Deudon E, Picard J (1986) Synthesis of sulfated proteoglycans throughout the cell cycle in smooth muscle cells from pig aorta. Exp Cell Res 166:416–426
Budu-Grajdeanu P, Schugart RC, Friedman A, Valentine C, Agarwal AK, Rovin BH (2008) A mathematical model of venous neointimal hyperplasia formation. Theor Biol Med Model 5:2
Bulelzai MAK, Dubbeldam JLA (2012) Long time evolution of atherosclerotic plaques. J Theor Biol 297:1–10
Byrne HM, Owen MR (2004) A new interpretation of the Keller–Segel model based on multiphase modelling. J Math Biol 49:604–626
Cai AQ, Landman KA, Hughes BD (2007) Multi-scale modeling of a wound healing cell migration assay. J Theor Biol 245:576–594
Chalmers AD, Cohen A, Bursill CA, Myerscough MR (2015) Bifurcation and dynamics in a mathematical model of early atherosclerosis. J Math Biol 71:1451–1480
Chalmers AD, Bursill CA, Myerscough MR (2017) Nonlinear dynamics of early atherosclerotic plaque formation may determine the efficacy of high density lipoproteins (HDL) in plaque regression. PLoS ONE 12:e0187674
Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jørgensen HF (2016) Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res 119:1313–1323
Chen CL, Liu IH, Fliesler SJ, Han X, Huang SS, Huang J (2007) Cholesterol suppresses cellular TGF-\(\beta \) responsiveness: implications in atherogenesis. J Cell Sci 120:3509–3521
Cilla M, Pena E, Martinez MA (2014) Mathematical modelling of atheroma plaque formation and development in coronary arteries. J R Soc Interface 11:20130866
Clarke MCH, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR (2006) Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med 12:1075–1080
Cobbold CA, Sherratt JA (2000) Mathematical modelling of nitric oxide activity in wound healing can explain keloid and hypertrophic scarring. J Theor Biol 204:257–288
Cohen A, Myerscough MR, Thompson RS (2014) Athero-protective effects of high density lipoproteins (HDL): an ODE model of the early stages of atherosclerosis. Bull Math Biol 76:1117–1142
Cumming BD, McElwain DLS, Upton Z (2010) A mathematical model of wound healing and subsequent scarring. J R Soc Interface 7:19–34
El Khatib N, Génieys S, Volpert V (2007) Atherosclerosis initiation modeled as an inflammatory process. Math Model Nat Phenom 2:126–141
El Khatib N, Génieys S, Kazmierczak B, Volpert V (2009) Mathematical modelling of atherosclerosis as an inflammatory disease. Philos Trans R Soc A 367:4877–4886
Evans DJW, Lawford PV, Gunn J, Walker D, Hose DR, Smallwood RH, Chopard B, Krafczyk M, Bernsdorf J, Hoekstra A (2008) The application of multiscale modelling to the process of development and prevention of stenosis in a stented coronary artery. Philos Trans R Soc A 366:3343–3360
Faggiotto A, Ross R (1984) Studies of hypercholesterolemia in the nonhuman primate II. Fatty streak conversion to fibrous plaque. Arteriosclerosis 4:341–356
Filipovic N, Teng Z, Radovic M, Saveljic I, Fotiadis D, Parodi O (2013) Computer simulation of three-dimensional plaque formation and progression in the carotid artery. Med Biol Eng Comput 51:607–616
Fok P (2012) Mathematical model of intimal thickening in atherosclerosis: vessel stenosis as a free boundary problem. J Theor Biol 314:23–33
Ford HZ, Byrne HM, Myerscough MR (2019) A lipid-structured model for macrophage populations in atherosclerotic plaques. J Theor Biol 479:48–63
Friedman A, Hao WR (2015) A mathematical model of atherosclerosis with reverse cholesterol transport and associated risk factors. Bull Math Biol 77:758–781
Fukumoto Y, Deguchi J, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M (2004) Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation 110:1953–1959
Funayama H, Ikeda U, Takahashi M, Sakata Y, Kitagawa SI, Takahashi YI, Masuyama JI, Furukawa Y, Miura Y, Kano S, Matsuda M, Shimada K (1998) Human monocyte-endothelial cell interaction induces platelet-derived growth factor expression. Cardiovasc Res 37:216–224
Garbey M, Casarin S, Berceli SA (2017) Vascular adaptation: pattern formation and cross validation between an agent based model and a dynamical system. J Theor Biol 429:149–163
Getz GS, Reardon CA (2012) Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol 32:1104–1115
Guo M, Cai Y, Yao X, Li Z (2018) Mathematical modeling of atherosclerotic plaque destabilization: role of neovascularization and intraplaque hemorrhage. J Theor Biol 450:53–65
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Immunol 6:508–519
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278:483–493
Haugh JM (2006) Deterministic model of dermal wound invasion incorporating receptor-mediated signal transduction and spatial gradient sensing. Biophys J 90:2297–2308
Hou G, Mulholland D, Gronska MA, Bendeck MP (2000) Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. Am J Pathol 156:467–476
Hsieh HJ, Li NQ, Frangos JA (1991) Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol 260:H642–H646
Huang JS, Olsen TJ, Huang SS (1988) The role of growth factors in tissue repair I. Platelet-derived growth factor. In: Clark RAF, Henson PM (eds) The molecular and cellular biology of wound repair. Plenum, New York, pp 243–251
Hubbard ME, Byrne HM (2013) Multiphase modelling of vascular tumour growth in two spatial dimensions. J Theor Biol 316:70–89
Islam MH, Johnston PR (2016) A mathematical model for atherosclerotic plaque formation and arterial wall remodelling. ANZIAM J 57:C320–C345
Jacobsen K, Lund MB, Shim J, Gunnersen S, Füchtbauer EM, Kjolby M, Carramolino L, Bentzon JF (2017) Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight 2:e95890
Klika V, Gaffney EA, Chen YC, Brown CP (2016) An overview of multiphase cartilage mechanical modelling and its role in understanding function and pathology. J Mech Behav Biomed 62:139–157
Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, Yu JC, Abe K, Martin PJ, Ross R, Betsholtz C, Giese NA, Raines EW (2002) Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol 161:1395–1407
Kubota K, Okazaki J, Louie O, Kent KC, Liu B (2003) TGF-\(\beta \) stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J Surg Res 109:43–50
Lally C, Prendergast P (2006) Simulation of in-stent restenosis for the design of cardiovascular stents. In: Holzapfel A, Ogden RW (eds) Mechanics of biological tissue. Springer, Berlin, pp 255–267
Lemon G, King JR, Byrne HM, Jensen OE, Shakesheff KM (2006) Mathematical modelling of engineered tissue growth using a multiphase porous flow mixture theory. J Math Biol 52:571–594
Lopes J, Adiguzel E, Gu S, Liu SL, Hou G, Heximer S, Assoian RK, Bendeck MP (2013) Type VIII collagen mediates vessel wall remodeling after arterial injury and fibrous cap formation in atherosclerosis. Am J Pathol 182:2241–2253
Lusis AJ (2000) Atherosclerosis. Nature 407:233–241
Lutgens E, de Muinck ED, Kitslaar PJEHM, Tordoir JHM, Wellens HJJ, Daemen MJAP (1999) Biphasic pattern of cell turnover characterises the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 41:473–479
Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJAP (2002) Transforming growth factor-\(\beta \) mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol 22:975–982
Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A (2001) Interleukin-18/Interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res 89:e41–e45
McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, Bush HL Jr (1995) Decreased type II/type I TGF-\(\beta \) receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-\(\beta \)1. J Clin Invest 96:2667–2675
McDougall S, Dallon J, Sherratt J, Maini P (2006) Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos Trans R Soc A 364:1385–1405
McKay C, McKee S, Mottram N, Mulholland T, Wilson S, Kennedy S, Wadsworth R (2004) Towards a model of atherosclerosis, Technical report. University of Strathclyde
Mel’nyk T (2019) Asymptotic analysis of a mathematical model of the atherosclerosis development. Int J Biomath 12:1950014
Menon SN, Flegg JA, McCue SW, Schugart RC, Dawson RA, McElwain DLS (2012) Modelling the interaction of keratinocytes and fibroblasts during normal and abnormal wound healing processes. Proc R Soc B 279:3329–3338
Moore K, Sheedy F, Fisher E (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13:709–721
Munro E, Patel M, Chan P, Betteridge L, Gallagher K, Schachter M, Wolfe J, Server P (1994) Effect of calcium channel blockers on the growth of human vascular smooth muscle cells derived from saphenous vein and vascular graft stenosis. J Cardiovasc Pharmacol 23:779–784
Nelson PR, Yamamura S, Kent KC (1996) Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J Vasc Surg 24:25–32
Nicolas M, Peña E, Malvè M, Martínez MA (2015) Mathematical modeling of the fibrosis process in the implantation of inferior vena cava filters. J Theor Biol 387:228–240
O’Dea RD, Osborne JM, El Haj AJ, Byrne HM, Waters SL (2013) The interplay between tissue growth and scaffold degradation in engineered tissue constructs. J Math Biol 67:1199–1225
Ogawa K, Chen F, Kuang C, Chen Y (2004) Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-\(\beta \) is mediated by a nuclear factor-\(\kappa \)B site. Biochem J 381:413–422
Olsen L, Sherratt JA, Maini PK (1995) A mechanochemical model for adult dermal wound contraction and the permanence of the contracted tissue displacement profile. J Theor Biol 177:113–128
Pappalardo F, Musumeci S, Motta S (2008) Modeling immune system control of atherogenesis. Bioinformatics 24:1715–1721
Parton A, McGilligan V, O’Kane M, Baldrick FR, Watterson S (2016) Computational modelling of atherosclerosis. Brief Bioinform 17:562–575
Pearson NC, Shipley RJ, Waters SL, Oliver JM (2014) Multiphase modelling of the influence of fluid flow and chemical concentration on tissue growth in a hollow fibre membrane bioreactor. Math Med Biol 31:393–430
Poston RN, Poston DRM (2007) Typical atherosclerotic plaque morphology produced in silico by an atherogenesis model based on self-perpetuating propagating macrophage recruitment. Math Model Nat Phenom 2:142–149
Preziosi L, Tosin A (2009) Multiphase modelling of tumour growth and extracellular matrix interaction: mathematical tools and applications. J Math Biol 58:625–656
Reifenberg K, Cheng F, Orning C, Crain J, Küpper I, Wiese E, Protschka M, Blessing M, Lackner KJ, Torzewski M (2012) Overexpression of TGF-\(\beta \)1 in macrophages reduces and stabilizes atherosclerotic plaques in ApoE-deficient mice. PLoS ONE 7:e40990
Risinger GM, Updike DL, Bullen E, Tomasek JJ, Howard EW (2010) TGF-\(\beta \) suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol 298:C191–C201
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Rutherford C, Martin W, Carrier M, Ånggård EE, Ferns GAA (1997) Endogenously elicited antibodies to platelet derived growth factor-BB and platelet cystolic protein inhibit aortic lesion development in the cholesterol-fed rabbit. Int J Exp Path 78:21–32
Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, Nishikawa S, Nishikawa SI, Kita T (2001) Functional blockade of platelet-derived growth factor receptor-\(\beta \) but not of receptor-\(\alpha \) prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation 103:2955–2960
Schachter M (1997) Vascular smooth muscle cell migration, atherosclerosis, and calcium channel blockers. Int J Cardiol 62:S85–S90
Silva T, Sequeira A, Santos RF, Tiago J (2016) Existence, uniqueness, stability and asymptotic behaviour of solutions for a mathematical model of atherosclerosis. Discrete Contin Dyn Syst Ser S 9:343–362
Singh NN, Ramji DP (2006) The role of transforming growth factor-\(\beta \) in atherosclerosis. J R Soc Interface 17:487–499
Tahir H, Niculescu I, Bona-Casas C, Merks RMH, Hoekstra AG (2015) An in silico study on the role of smooth muscle cell migration in neointimal formation after coronary stenting. Cytokine Growth Factor Rev 12:20150358
Toma I, McCaffrey TA (2012) Transforming growth factor-\(\beta \) and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 347:155–175
Urschel K, Cicha I (2015) TNF-\(\alpha \) in the cardiovascular system: from physiology to therapy. Int J Interferon Cytokine Mediat Res 7:9–25
Vaday GG, Schor H, Rahat MA, Lahat N, Lider O (2001) Transforming growth factor-\(\beta \) suppresses tumor necrosis factor \(\alpha \)-induced matrix metalloproteinase-9 expression in monocytes. J Leukoc Biol 69:613–621
Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA (2015) Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 35:535–546
Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn M (1990) Recombinant latent transforming growth factor \(\beta \)1 has a longer plasma half-life in rats than active transforming growth factor \(\beta \)1, and a different tissue distribution. J Clin Invest 86:1976–1984
Wakefield LM, Letterio JJ, Chen T, Danielpour D, Allison RS, Pai LH, Denicoff AM, Noone MH, Cowan KH, O’Shaughnessy JA, Sporn M (1995) Transforming growth factor-\(\beta \)1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clin Cancer Res 1:129–136
Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M (2015) Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132:1909–1919
Watson MG, Byrne HM, Macaskill C, Myerscough MR (2018) A two-phase model of early fibrous cap formation in atherosclerosis. J Theor Biol 456:123–136
World Health Organization (2017) Cardiovascular diseases fact sheet. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed April 2019
Yang Y, Jäger W, Neuss-Radu M, Richter T (2016) Mathematical modeling and simulation of the evolution of plaques in blood vessels. J Math Biol 72:973–996
Zahedmanesh H, Van Oosterwyck H, Lally C (2014) A multi-scale mechanobiological model of in-stent restenosis: deciphering the role of matrix metalloproteinase and extracellular matrix changes. Comput Methods Biomech Biomed Eng 17:813–828
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael G. Watson, Charlie Macaskill and Mary R. Myerscough acknowledge funding from an Australian Research Council Discovery Grant (DP160104685).
Rights and permissions
About this article
Cite this article
Watson, M.G., Byrne, H.M., Macaskill, C. et al. A multiphase model of growth factor-regulated atherosclerotic cap formation. J. Math. Biol. 81, 725–767 (2020). https://doi.org/10.1007/s00285-020-01526-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-020-01526-6
Keywords
- Atherosclerosis
- Multiphase model
- Smooth muscle cells
- Platelet-derived growth factor
- Transforming growth factor-\(\beta \)