Abstract
Purpose
An observational study was conducted to evaluate the pharmacokinetics of venetoclax and its impact on the efficacy and safety for Japanese patients with acute myeloid leukemia (AML) treated with venetoclax and azacitidine therapy.
Methods
The association between the plasma concentration, after the first cycle of azacitidine and venetoclax therapy, and the efficacy and safety was evaluated in 33 patients with untreated or relapsed/refractory AML.
Results
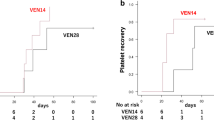
Full dose of venetoclax was administered to all patients. Venetoclax treatment was 28 day long in 82% of patients; the relative dose intensity of azacitidine was 82%. Trough concentration was significantly higher among patients with complete remission (CR) and CR with incomplete hematologic recovery (CRi) than those with the morphologic leukemia-free state and partial remission, and no response groups (P = 0.01). Median duration of grade 3 neutropenia was 28 days (range 8–46 days). Area under the concentration–time curve (AUC0–24) was significantly higher among patients with protracted grade 3 neutropenia (≥ 28 days) than those with a shorter duration (< 28 days) (P = 0.03); multivariate analysis revealed that a higher AUC0–24 was a significant predictor of a longer duration of neutropenia (odds ratio 54.3, P = 0.007).
Conclusion
Plasma concentrations of venetoclax were variable in Japanese patients with AML. Higher plasma concentrations were associated with CR/CRi and protracted grade 3 neutropenia. Therefore, it is essential to adjust the duration of venetoclax administration based on individual pharmacokinetic data to limit total drug exposure, reduce severe neutropenia, and achieve higher efficacy.



Similar content being viewed by others
References
Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C (2015) Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol 94(7):1127–1138
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH et al (2020) Azacitidine and Venetoclax in previously untreated Acute Myeloid Leukemia. N Engl J Med 383(7):617–629
Pratz KW, DiNardo CD, Selleslag D, Li J, Yamamoto K, Konopleva M et al (2022) Postremission Cytopenia management in patients with acute myeloid leukemia treated with venetoclax and azacitidine in VIALE-A. Am J Hematol 97(11):E416–e19
Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK et al (2015) Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 7(279):279ra40
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF et al (2016) Targeting BCL2 with Venetoclax in Relapsed Chronic lymphocytic leukemia. N Engl J Med 374(4):311–322
Cheung TT, Salem AH, Menon RM, Munasinghe WP, Bueno OF, Agarwal SK (2018) Pharmacokinetics of the BCL-2 inhibitor venetoclax in healthy Chinese subjects. Clin Pharmacol Drug Dev 7(4):435–440
Brackman D, Eckert D, Menon R, Salem AH, Potluri J, Smith BD et al (2022) Venetoclax exposure-efficacy and exposure-safety relationships in patients with treatment-naïve acute myeloid leukemia who are ineligible for intensive chemotherapy. Hematol Oncol 40(2):269–279
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al (2003) Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in Acute myeloid leukemia. J Clin Oncol 21(24):4642–4649
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H et al (2022) Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140(12):1345–1377
Fukuda N, Kobayashi T, Sato H, Akamine Y, Takahashi N, Miura M (2022) Quantitation of Venetoclax in Human plasma by high-performance liquid chromatography with Ultraviolet Detection. J Chromatogr Sci. Oct 31
Takahashi N, Miura M, Scott SA, Kagaya H, Kameoka Y, Tagawa H et al (2010) Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J Hum Genet 55(11):731–737
Kobayashi T, Miura M, Abumiya M, Akamine Y, Ito F, Takahashi N (2019) Influence of ABCB1 polymorphisms on the pharmacokinetics and toxicity of lenalidomide in patients with multiple myeloma. Med Oncol 36(6):55
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48(3):452–458
Agarwal S, Gopalakrishnan S, Mensing S, Potluri J, Hayslip J, Kirschbrown W et al (2019) Optimizing venetoclax dose in combination with low intensive therapies in elderly patients with newly diagnosed acute myeloid leukemia: an exposure-response analysis. Hematol Oncol 37(4):464–473
Yamamoto K, Shinagawa A, DiNardo CD, Pratz KW, Ishizawa K, Miyamoto T et al (2022) Venetoclax plus azacitidine in Japanese patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy. Jpn J Clin Oncol 52(1):29–38
Taniguchi S, Yamauchi T, Choi I, Fukuhara N, Potluri J, Salem AH et al (2021) Venetoclax in combination with azacitidine in Japanese patients with acute myeloid leukaemia: phase 1 trial findings. Jpn J Clin Oncol 51(6):857–864
Jones AK, Freise KJ, Agarwal SK, Humerickhouse RA, Wong SL, Salem AH (2016) Clinical predictors of Venetoclax Pharmacokinetics in Chronic lymphocytic leukemia and Non-hodgkin’s Lymphoma patients: a Pooled Population Pharmacokinetic Analysis. Aaps j 18(5):1192–1202
Agarwal SK, Hu B, Chien D, Wong SL, Salem AH (2016) Evaluation of Rifampin’s Transporter Inhibitory and CYP3A Inductive effects on the pharmacokinetics of Venetoclax, a BCL-2 inhibitor: results of a single- and multiple-dose study. J Clin Pharmacol 56(11):1335–1343
Agarwal SK, DiNardo CD, Potluri J, Dunbar M, Kantarjian HM, Humerickhouse RA et al (2017) Management of Venetoclax-Posaconazole Interaction in Acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther 39(2):359–367
Agarwal SK, Salem AH, Danilov AV, Hu B, Puvvada S, Gutierrez M et al (2017) Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-hodgkin lymphoma. Br J Clin Pharmacol 83(4):846–854
Mehta SV, Shukla SN, Vora HH (2013) Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: its correlation with FLT3. Neoplasma 60(6):666–675
Vachhani P, Flahavan EM, Xu T, Ma E, Montez M, Gershon A et al (2022) Venetoclax and Hypomethylating agents as First-line treatment in newly diagnosed patients with AML in a Predominately Community setting in the US. Oncologist 27(11):907–918
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Study design: T.K., Y.K., N.T.Patient enrollment: T.K., H.S., W.K., F.I., K.T., A.W., N.F., I.K.Measurement of plasma concentration of venetoclax and genoty**: M.M., Y.F.Collection and assembly of data: T.K., H.S.Data Analyses (including statistical): T.K., H.S., M.M.Manuscript writing: T.K., H.S.Final approval of the manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and informed consent
The multicenter prospective observational study was conducted in Akita prefecture, Japan. This study enrolled patients with AML who were judged ineligible for intensive chemotherapy by the attending physician. This study was conducted in accordance with the 1964 Helsinki Declaration and approved by the Ethics Committee of Akita University (August 6, 2021/No. 2696). Written informed consent was obtained from all participants before enrollment.
Conflict of interests
Naoto Takahashi received honoraria from Otsuka, Novartis, and received research funding from Otsuka, Novartis, Astellas, Asahikasei Pharma, and Ono Pharmaceutical.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, T., Sato, H., Miura, M. et al. Overexposure to venetoclax is associated with prolonged-duration of neutropenia during venetoclax and azacitidine therapy in Japanese patients with acute myeloid leukemia. Cancer Chemother Pharmacol (2024). https://doi.org/10.1007/s00280-024-04673-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00280-024-04673-5