Abstract
Purpose
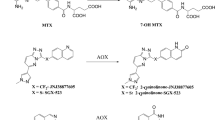
Benzaldehyde dimethane sulfonate (DMS612, NSC281612, BEN) is an alkylator with activity against renal cell carcinoma, currently in phase I trials. In blood, BEN is rapidly metabolized into its highly reactive carboxylic acid (BA), presumably the predominant alkylating species. We hypothesized that BEN is metabolized to BA by aldehyde dehydrogenase (ALDH) and aimed to increase BEN exposure in blood and tissues by inhibiting ALDH with disulfiram, thereby shifting BA production from blood to tissues.
Methods
Female CD2F1 mice were dosed with 20 mg/kg BEN iv alone or 24 h after 300 mg/kg disulfiram ip. BEN, BA, and metabolites were quantitated in plasma and urine, and toxicities were assessed.
Results
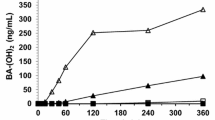
BEN had a plasma t½ <5 min and produced at least 12 products. The metabolite half-lives were <136 min. Disulfiram increased BEN plasma exposure 368-fold (AUC0–inf from 0.11 to 40.5 mg/L min), while plasma levels of BA remained similar. Urinary BEN excretion increased (1.0–1.5 % of dose), while BA excretion was unchanged. Hematocrit, white blood cell counts, and percentage lymphocytes decreased after BEN administration. Coadministration of disulfiram appeared to enhance these effects. Profound liver pathology was observed in mice treated with disulfiram and BEN.
Conclusions
BEN plasma concentrations increased after administration of disulfiram, suggesting that ALDH mediates the rapid metabolism of BEN in vivo, which may explain the increased toxicity seen with BEN after administration of disulfiram. Our results suggest that the coadministration of BEN with drugs that inhibit ALDH to patients that are ALDH deficient may cause liver damage.




Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353(23):2477–2490
Facchini G, Perri F, Caraglia M, Pisano C, Striano S, Marra L, Fiore F, Aprea P, Pignata S, Iaffaioli RV (2009) New treatment approaches in renal cell carcinoma. Anticancer Drugs 20(10):893–900
Rini BI, Atkins MB (2009) Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 10(10):992–1000
Rini BI (2009) Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol 27(19):3225–3234
Reeves DJ, Liu CY (2009) Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharmacol 64(1):11–25
Mertins SD, Myers TG, Holbeck SL, Medina-Perez W, Wang E, Kohlhagen G, Pommier Y, Bates SE (2004) In vitro evaluation of dimethane sulfonate analogues with potential alkylating activity and selective renal cell carcinoma cytotoxicity. Mol Cancer Ther 3(7):849–860
Mertins S (2011) Treating renal cancer using a 4-[bis[2-[(methylsulfonyl)oxy]ethyl]amino]-2-methyl-benzaldehyde. Patent Application Publication, United States
Carter J (2005) In vivo efficacy of an aldehyde degradation product of dimethane sulfonate (NSC 281612) in an orthotopic RXF-393 human renal tumor model. In: Proceedings of the American Association of Cancer Research, 2005. pp 322–323
Parise RA, Anyang BN, Eiseman JL, Egorin MJ, Covey JM, Beumer JH (2012) Formation of active products of benzaldehyde dimethane sulfonate (NSC 281612, DMS612) in human blood and plasma and their activity against renal cell carcinoma lines. Cancer Chemother Pharmacol. doi:10.1007/s00280-012-1980-1
Marchitti SA, Brocker C, Stagos D, Vasiliou V (2008) Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol 4(6):697–720
Tottmar O, Hellstrom E (1983) Aldehyde dehydrogenase in blood: a sensitive assay and inhibition by disulfiram. Pharmacol Biochem Behav 18(Suppl 1):103–107
Beumer JH, Franke NE, Tolboom R, Buckle T, Rosing H, Lopez-Lazaro L, Schellens JH, Beijnen JH, van Tellingen O (2010) Disposition and toxicity of trabectedin (ET-743) in wild-type and mdr1 gene (P-gp) knock-out mice. Invest New Drugs 28(2):145–155. doi:10.1007/s10637-009-9234-8
Bailer AJ (1988) Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm 16(3):303–309
Pratt-Hyatt M, Lin HL, Hollenberg PF (2010) Mechanism-based inactivation of human CYP2E1 by diethyldithocarbamate. Drug Metab Dispos Biol Fate Chem 38(12):2286–2292. doi:10.1124/dmd.110.034710
Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B, Chang LJ (2012) The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact 195(1):52–60. doi:10.1016/j.cbi.2011.10.007
Chippendale TW, Hu B, El Haj AJ, Smith D (2012) A study of enzymatic activity in cell cultures via the analysis of volatile biomarkers. Analyst. doi:10.1039/c2an35815h
Kong D, Kotraiah V (2012) Modulation of aldehyde dehydrogenase activity affects (±)-4-hydroxy-2E-nonenal (HNE) toxicity and HNE-protein adduct levels in PC12 cells. J Mol Neurosci 47(3):595–603. doi:10.1007/s12031-011-9688-y
Kotraiah V, Pallares D, Toema D, Kong D, Beausoleil E (2012) Identification of aldehyde dehydrogenase 1A1 modulators using virtual screening. J Enzyme Inhib Med Chem. doi:10.3109/14756366.2011.653353
Stagos D, Chen Y, Brocker C, Donald E, Jackson BC, Orlicky DJ, Thompson DC, Vasiliou V (2010) Aldehyde dehydrogenase 1B1: molecular cloning and characterization of a novel mitochondrial acetaldehyde-metabolizing enzyme. Drug Metab Dispos Biol Fate Chem 38(10):1679–1687. doi:10.1124/dmd.110.034678
Povirk LF, Shuker DE (1994) DNA damage and mutagenesis induced by nitrogen mustards. Mutat Res 318(3):205–226
Hall AG, Tilby MJ (1992) Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev 6(3):163–173
Ciarimboli G, Holle SK, Vollenbrocker B, Hagos Y, Reuter S, Burckhardt G, Bierer S, Herrmann E, Pavenstadt H, Rossi R, Kleta R, Schlatter E (2010) New clues for nephrotoxicity induced by ifosfamide: preferential renal uptake via the human organic cation transporter 2. Mol Pharm
Schulz C, Boeck S, Heinemann V, Stemmler HJ (2009) UGT1A1 genoty**: a predictor of irinotecan-associated side effects and drug efficacy? Anticancer Drugs 20(10):867–879
Karamanakos PN, Pappas P, Boumba VA, Thomas C, Malamas M, Vougiouklakis T, Marselos M (2007) Pharmaceutical agents known to produce disulfiram-like reaction: effects on hepatic ethanol metabolism and brain monoamines. Int J Toxicol 26(5):423–432. doi:10.1080/10915810701583010
Acknowledgments
We thank Diane Mazzei and her colleagues at the University of Pittsburgh Animal Facility for their expert assistance, and the University of Pittsburgh Cancer Institute Hematology/Oncology Writing Group for constructive suggestions regarding the manuscript. Also, we like to thank Dr. Joseph M. Covey at the Toxicology and Pharmacology Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, for his intellectual input. We would like to thank Dr. Merrill Egorin for his help and guidance on conducting the experimentation that was required for this manuscript. He was a great mentor, colleague, and friend, and he will not be forgotten. This work was supported by the National Cancer Institute [Contract N01-CM-52202] and [Grants U01-CA099168, P30-CA47904]. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parise, R.A., Beumer, J.H., Clausen, D.M. et al. Effects of the aldehyde dehydrogenase inhibitor disulfiram on the plasma pharmacokinetics, metabolism, and toxicity of benzaldehyde dimethane sulfonate (NSC281612, DMS612, BEN) in mice. Cancer Chemother Pharmacol 72, 1195–1204 (2013). https://doi.org/10.1007/s00280-013-2296-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2296-5