Abstract
Purpose
The dorsolateral prefrontal cortex (DLPFC) is a cortical area involved in higher cognitive functions, and at the center of the pathophysiology of mental disorders such as depression and schizophrenia. Considering these major roles and the development of deep brain stimulation, the object of this study was to assess the patterns of connectivity of the DLPFC with its main subcortical relay, the thalamus, with the help of probabilistic tractography.
Methods
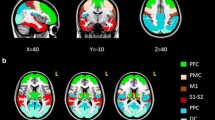
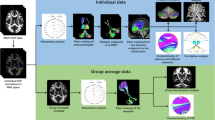
We used T1-weighted imaging and diffusion data from 18 subjects from the Human Connectome Project. The DLPFC and the thalamic nuclear groups were defined using the combination of atlases, sulcogyral anatomy and cytoarchitectonic data. Probabilistic tractography was performed from the DLPFC to the thalamus. The patterns of connectivity were assessed using two indexes: (1) a connectivity index (CI) which evaluate the strength of connection (2) an asymmetry index (AI) which explores the inter-hemispheric variability.
Results
The analysis of CI showed significant connections between the DLPFC and the dorsomedial nuclei (p < 0.05), the anterior nuclear groups (p < 0.05) and the right centromedian nucleus (p < 0.05). No link was found between handedness and AI (p > 0.05). Most of subjects (15/18) had a right predominance of the thalamo cortical connections of the DLPFC.
Conclusions
Probabilistic tractography appears as a valuable non-invasive tool for the exploration of the thalamocortical connections between the dorsolateral prefrontal cortex and thalamic nuclei. It allowed to show different inter-hemispheric patterns of connectivity, and highlighted the centromedian nucleus as a key subcortical relay of executive functions.



Similar content being viewed by others
References
Balconi M (2013) Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull 29:381–389
Bardinet E, Bhattacharjee M, Dormont D, Pidoux B, Malandain G, Schüpbach M, Ayache N, Cornu P, Agid Y, Yelnik J (2009) A three-dimensional histological atlas of the human basal ganglia. II Atlas deformation strategy and evaluation in deep brain stimulation for Parkinson disease. J Neurosurg 110:208–219
Carmel PW (1970) Efferent projections of the ventral anterior nucleus of the thalamus in the monkey. Am J Anatomy 128:159–183
Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, Yang D, Mu J, Zhu D, Zou D, **e P (2013) Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomized trials. Psychiatry Res 210:1260–1264
D’Albis T, Haegelen C, Essert C, Fernandez-Vidal S, Lalys F, Jannin P (2014) PyDBS: an automated image processing workflow for deep brain stimulation surgery. Int J Comput Assist Radiol Surg 10:117–128
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Buckner RL, Blacker D, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Drevets WC, Savitz J, Trimble M (2008) The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Krishnamurthy KB, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, SANTE Study Group (2010) Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51:899–908
Fischl B (2012) FreeSurfer. NeuroImage 62:774–781
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
George MS, Taylor JJ, Short EB (2013) The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 26:13–18
Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R (2014) Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA 111:823–828
Jakab A, Blanc R, Berényi EL (2012) Map** changes of in vivo connectivity patterns in the human mediodorsal thalamus: correlations with higher cognitive and executive functions. Brain Imaging Behav 6:472–483
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62:782–783
Kito S, Jung J, Kobayashi T, Koga Y (2009) Fiber tracking of white matter integrity connecting the mediodorsal nucleus of the thalamus and the prefrontal cortex in schizophrenia: a diffusion tensor imaging study. Eur Psychiatry 24:269–274
Klein JC, Rushworth MFS, Behrens TEJ, Mackay CE, de Crespigny AJ, D’Arcueil H, Johansen-Berg H (2012) Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage 52:555–564
Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Styner M, Gilmore JH (2014) Impact of sex and gonadal steroids on neonatal brain structure. Cereb Cortex 24:2721–2731
Lalys F, Haegelen C, Mehri M, Drapier S, Verin M, Jannin P (2013) Anatomo-clinical atlases correlate clinical data and electrode contact coordinates: application to subthalamic deep brain stimulation. J Neurosci Methods 212:297–307
Magro E, Moreau T, Seizeur R, Zemmoura I, Gibaud B, Morandi X (2014) Connectivity within the primary motor cortex: a DTI tractography study. Surg Radiol Anat 36:125–135
Martin JL, Barbanoj JM, Pérez V, Sacristan M (2003) Transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder. Cochrane Database Syst Rev
Mashour GA, Walker EE, Mazurta RL (2005) Psychosurgery: past, present, and future. Brain Res Brain Res Rev 48:409–419
Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS (2006) Correlation between volume of the pulvinar, centromedian, and mediodorsal nuclei and cortical Brodmann’s areas in schizophrenia. Neurosci Lett 392:16–21
Nahas Z, Molloy MA, Hughes PL, Oliver NC, Arana GW, Risch SG, George MS (1999) Repetitive transcranial magnetic stimulation: perspectives for application in the treatment of bipolar and unipolar disorders. Bipolar Disord 1:73–80
Nauczyciel C, Hellier P, Morandi X, Blestel S, Drapier D, Ferre JC, Barillot C, Millet B (2011) Assessment of standard coil positioning in TMS for depression. Psychiatry Res 186:232–238
Pasnicu A, Denoyer Y, Haegelen C, Pasqualini E, Biraben A (2013) Modulation of paroxysmal activity in focal cortical dysplasia by centromedian thalamic nucleus stimulation. Epilepsy Res 104:264–268
Petrides M (2005) Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360:781–795
Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN (2012) The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex 48:46–57
Rajkowska G, Goldman-Rakic PS (1995) Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. remap** of areas 9 and 46 unsing quantitative criteria. Cereb Cortex 5:307–322
Ray JP, Price JL (1993) The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 337:1–31
Salerian AJ, Altar CA (2012) The prefrontal cortex influence over subcortical and limbic regions governs antidepressant response by N = H/(M + R). Psychiatry Res 204:1–12
Sallet J, Mars RB, Nooman MA, Neubert FX, Jbabdi S, O’Reilly JX, Filippini N, Thomas AG, Rushworth MF (2013) The organization of dorsal frontal cortex in humans and macaques. The J Neurosci 33:12255–12274
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull strip** problem in MRI. Neuroimage 22:1060–1075
Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, WU-Minn HCP Consortium (2013) The WU-Minn human connectome project : an overview. NeuroImage 80:62–79
**ao D, Zikopoulos B, Barbas H (2009) Lamina and molecular organization of prefrontal projections to multiple thalamic nuclei. Neuroscience 161:1067–1081
Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tandé D, Karachi C, Ayache N, Cornu P, Agid Y (2007) A three-dimensional, histological and deformable atlas of the human basal ganglia. I. atlas construction on immunohistochemical and MRI data. Neuroimage 34:618–638
Acknowledgments
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We wanted to thank Cécile-Marie Le Reste for her help in revising the language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Le Reste, PJ., Haegelen, C., Gibaud, B. et al. Connections of the dorsolateral prefrontal cortex with the thalamus: a probabilistic tractography study. Surg Radiol Anat 38, 705–710 (2016). https://doi.org/10.1007/s00276-015-1603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-015-1603-8