Abstract
Background
Immune checkpoint inhibitors (ICIs) are a first-line treatment for various metastatic solid tumors. Pneumonitis is a potentially devastating complication of ICI treatment and a leading cause of ICI-related mortality. Here, we evaluate whether abnormal pre-treatment pulmonary function tests (PFTs) or interstitial abnormalities on computed tomography of the chest (CT chest) prior to ICI are associated with the development of ICI-pneumonitis (ICI-p).
Methods
We conducted a retrospective cohort study of consecutive patients who received at least one dose of ICI from 2011 to 2017 at The Ohio State University. Potential risk factors for ICI-p, including abnormal PFTs and CT chest, were recorded. These risk factors were compared between patients with and without pneumonitis.
Results
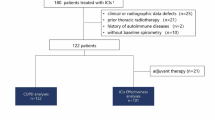
In total, 1097 patients were included, 46 with ICI-p and 1051 without. Ninety percent of patients had pre-treatment chest imaging, while only 10% had pre-treatment PFTs. On multivariable analysis, interstitial abnormalities and reduced total lung capacity (TLC) were significantly associated with development of ICI-p (hazard ratio of 42.42 [95% CI; 15.04–119.67] and hazard ratio of 4.04 [95% CI; 1.32–12.37]), respectively. No other PFT abnormality was associated with increased risk of ICI-p. There was no significant difference in overall survival in patients who did or did not develop ICI-p (p = 0.332).
Conclusions
Pre-existing interstitial abnormalities on CT chest and reduced TLC were strongly associated with develo** ICI-p. Prospective studies are warranted to further explore the role of PFTs as a potential tool for identifying patients at highest risk for develo** ICI-p.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
18 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00262-023-03391-w
References
Robert C (2020) A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. https://doi.org/10.1038/s41467-020-17670-y
Tanaka A, Sakaguchi S (2019) Targeting treg cells in cancer immunotherapy. Eur J Immunol 49(8):1140–1146
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375(19):1823–1833
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM (2013) Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 378(14):1277–1290
Yang S, Yu KH, Palmer N, Fox K, Kou SC, Kohane IS (20ol19) Autoimmune effects of lung cancer immunotherapy revealed by data-driven analysis on a nationwide cohort. Clin Pharmacol Ther 107(2):388–396
Boland P, Pavlick AC, Weber J, Sandigursky S (2020) Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J Immunother Cancer 8(1):e000356
Naidoo J, Naidoo J, Brahmer JR (2016) Immunotherapy for lung cancer: no longer an abstract concept. Semin Respir Crit Care Med 37(05):771–782
Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS (2016) Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer a systematic review and meta-analysis. JAMA Oncol 2(12):1607–1616
Wu J, Hong D, Zhang X, Lu X, Miao J (2017) PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Sci Rep. https://doi.org/10.1038/srep44173
Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y (2018) Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung cancer Amsterdam Netherlands 125:212–217
Suresh K, Voong KR, Shankar B et al (2018) Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol 13(12):1930–1939
Zhai X, Zhang J, Tian Y et al (2020) The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med 17(3):599–611
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J (2017) Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35(7):709–717
Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J (2017) Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer. Chest 152(2):271–281
Antonia SJ, Villegas A, Daniel D et al (2017) Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377(20):1919–1929
Shimoji K, Masuda T, Yamaguchi K et al (2020) Association of preexisting interstitial lung abnormalities with immune checkpoint inhibitor-induced interstitial lung disease among patients with nonlung cancers. JAMA Netw Open 3(11):e2022906
Kanai O, Kim YH, Demura Y, Kanai M, Ito T, Fujita K (2018) Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease Nivolumab in patients with ILD. Thoracic Cancer 9(7):847–855
Suzuki Y, Karayama M, Uto T et al (2020) Assessment of Immune-Related Interstitial Lung Disease in Patients With NSCLC Treated with Immune Checkpoint Inhibitors: A Multicenter Prospective Study. J Thorac Oncol 15(8):1317–1327
Sears CR, Peikert T, Possick JD, Naidoo J, Nishino M, Patel SP (2019) Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis an official American thoracic society research statement. Am J Respir Crit care Med 200(6):31–43
Li F, Zhou Z, Wu A et al (2018) Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiat Oncol 13(1):82
Ozawa Y, Abe T, Omae M et al (2015) Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: retrospective analysis of patients with lung cancer. PLoS ONE 10(10):e0140437
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap) a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 42(2):377–381
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inf 95:103208
Li M, Spakowicz D, Zhao S et al (2020) Brief report: inhaled corticosteroid use and the risk of checkpoint inhibitor pneumonitis in patients with advanced cancer. Cancer Immunol Immunother CII 69(11):2403–2408
Shankar B, Zhang J, Naqash AR et al (2020) Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol 6(12):1952–1956
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL (2019) Standardization of spirometry 2019 update an official American thoracic society and european respiratory society technical statement. Am J Respir Crit Care Med 200(8):e70–e88
Sterk PJ (2004) Lets not forget: the GOLD criteria for COPD are based on post-bronchodilator FEV1. Eur Respir J 23(4):497–498
Nishino M, Brais LK, Brooks NV, Hatabu H, Kulke MH, Ramaiya NH (1990) Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: a radiographic pattern-based approach. Eur J Cancer 53:163–170
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y (2008) Interstitial lung disease in Japanese patients with lung cancer a cohort and nested case-control study. Am J Respir Crit Care Med 177(12):1348–1357
Adegunsoye A, Vij R, Noth I (2019) Integrating Genomics Into Management of Fibrotic Interstitial Lung Disease. Chest 155(5):1026–1040
Desai O, Winkler J, Minasyan M, Herzog EL (2018) The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front Med (Lausanne) 5:43
Suresh K, Naidoo J, Zhong Q et al (2019) The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest 129(10):4305–4315
Reuss JE, Brigham E, Psoter KJ et al (2021) Pretreatment Lung Function and Checkpoint Inhibitor Pneumonitis in NSCLC. JTO Clin Res Rep 2(10):100220
Orens JB, Kazerooni EA, Martinez FJ et al (1995) The sensitivity of high-resolution CT in detecting idiopathic pulmonary fibrosis proved by open lung biopsy. A prospective study. Chest. 108(1):109–115
Flaherty KR, Martinez FJ (2001) Diagnosing interstitial lung disease: a practical approach to a difficult problem. Cleve Clin J Med 68(1):33–34
Morganroth PA, Kreider ME, Okawa J et al (2010) Interstitial lung disease in classic and skin-predominant dermatomyositis: a retrospective study with screening recommendations. Arch Dermatol 146(7):729–738
Zhu S, Fu Y, Zhu B, Zhang B, Wang J (2020) Pneumonitis Induced by Immune Checkpoint Inhibitors: From Clinical Data to Translational Investigation. Front Oncol 10:1785
Funding
This study was supported by the National Institutes of Health (P30CA016058). Research support provided by the REDCap project and The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, Grant UL1TR002733). Dr. Owen is supported by the LUNGevity Foundation Career Development Award. Dr. Presley is supported by the National Institute of Aging (C.J.P., 1K76AG074923-01) the following provide research funding to the Institution: BMS, Genentech, Pfizer, AbbVie, Merck, Elevation Oncology, Onc.ai.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, data analysis, and manuscript preparation were performed by AW, MR, DO, and KH. Database building and data collection were done by ML, GL, GO, KK, and CP. Statistical analysis was performed by SZ and LW. Imaging review was done by VE and JW. All authors reviewed the manuscript and approved the final submission.
Corresponding author
Ethics declarations
Conflict of interests
Dr. Otterson serves as a consultant on the Data Safety Monitoring Board on Beigene and Novocure. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study protocol was reviewed and approved by the Ohio State Institutional Review Board (2016C0070), and a waiver of informed consent was granted due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, A., Riley, M., Zhao, S. et al. Association between pre-treatment chest imaging and pulmonary function abnormalities and immune checkpoint inhibitor pneumonitis. Cancer Immunol Immunother 72, 1727–1735 (2023). https://doi.org/10.1007/s00262-023-03373-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03373-y