Abstract
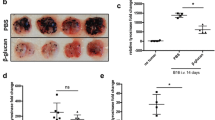
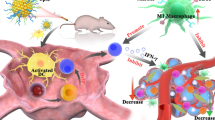
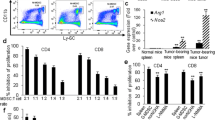
In the tumor microenvironment (TME), one of the major functions of tumor-recruited CD11b+ cells are the suppression of the T-cell-mediated anti-tumor immune response. β-glucan could convert the phenotype of tumor-recruited CD11b+ cells from the suppressive to the promotive, and enhanced their anti-tumor effects. However, β-glucan could enhance the PD-1/PD-L1 expression on CD11b+ cells, while PD-1 could inhibit macrophage phagocytosis and PD-L1 could induce a co-inhibitory signal in T-cells and lead to T-cell apoptosis and anergy. These protumor effects may be reversed by PD-1/PD-L1 block therapy. In the present study, we focused on the efficacy of β-glucan anti-tumor therapy combined with anti-PD-L1 mAb treatment, and the mechanism of their synergistic effects could be fully verified. We verified the effect of β-glucan (i.e., inflammatory cytokine secretion of TNF-α, IL-12, IL-6, IL-1β and the expression of immune checkpoint PD-1/PD-L1) in naïve mouse peritoneal exudate CD11b+ cells. In our mouse melanoma model, treatment with a PD-L1 blocking antibody with β-glucan synergized tumor regression. After treatment with β-glucan and anti-PD-L1 mAb antibody, tumor infiltrating leukocyte (TILs) not only showed a competent T-cell function (CD107a, perforin, IL-2, IFN-γ and Ki67) and CTL population, but also showed enhanced tumor-recruited CD11b+ cell activity (IL-12, IL-6, IL-1β and PD-1). This effect was also verified in the peritoneal exudate CD11b+ cells of tumor-bearing mice. PD-1/PD-L1 blockade therapy enhanced the β-glucan antitumor effects via the blockade of tumor-recruited CD11b+ cell immune checkpoints in the melanoma model.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AP-BG:
-
Aureobasidium pullulans-Produced β-glucan
- CTLs:
-
Cytotoxic T-cells
- MDSC:
-
Myeloid-derived suppressor cells
- MFI:
-
Mean fluorescence intensity
- PECs:
-
Peritoneal exudate cells
- PMA:
-
Phorbol 12-myristate 13-acetate
- TADC:
-
Tumor-associated DCs
- TAM:
-
Tumor-associated macrophages
- TGI:
-
Tumor growth inhibition
- TIL:
-
Tumor-infiltrating leukocytes
- TIM:
-
Tumor-infiltrating myeloid cells
- TME:
-
Tumor microenvironment
References
Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D (2011) Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 121(10):4015–4029. https://doi.org/10.1172/JCI45862
Schar** NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM (2016) The Tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45(2):374–388. https://doi.org/10.1016/j.immuni.2016.08.009
Park JA, Wang L, Cheung NV (2001) Modulating tumor infiltrating myeloid cells to enhance bispecific antibody-driven T cell infiltration and anti-tumor response. J Hematol Oncol 14(1):142. https://doi.org/10.1186/s13045-021-01156-5
Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T, Kunisada T, Ozaki T, Udono H (2019) Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol 31(4):187–198. https://doi.org/10.1093/intimm/dxy079
Kalafati L, Kourtzelis I, Schulte-Schrep** J, Li X, Hatzioannou A, Grinenko T, Hagag E, Sinha A, Has C, Dietz S et al (2020) Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183(3):771-785 e12. https://doi.org/10.1016/j.cell.2020.09.058
Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML et al (2010) Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115(7):1461–1471. https://doi.org/10.1182/blood-2009-08-237412
Chan GC, Chan WK, Sze DM (2009) The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol 2:25. https://doi.org/10.1186/1756-8722-2-25
Vetvicka V, Vannucci L, Sima P, Richter J (2019) Beta Glucan: Supplement or Drug? From laboratory to clinical trials. Molecules 24(7):1251. https://doi.org/10.3390/molecules24071251
Murphy EJ, Rezoagli E, Major I, Rowan NJ, Laffey JG (2020) Beta-glucan metabolic and immunomodulatory properties and potential for clinical application. J Fungi (Basel) 6(4):356. https://doi.org/10.3390/jof6040356
Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S et al (2017) Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med 23(5):556–567. https://doi.org/10.1038/nm.4314
Moriya N, Moriya Y, Nomura H, Kusano K, Asada Y, Uchiyama H, Park EY, Okabe M (2014) Improved β-glucan yield using an Aureobasidium pullulans M-2 mutant strain in a 200-L pilot scale fermentor targeting industrial mass production. Biotechnol Bioprocess Eng 18(6):1083–1089
Muramatsu D, Iwai A, Aoki S, Uchiyama H, Kawata K, Nakayama Y, Nikawa Y, Kusano K, Okabe M, Miyazaki T (2012) beta-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 7(7):e41399. https://doi.org/10.1371/journal.pone.0041399
Shui Y, Hu X, Hirano H, Kusano K, Tsukamoto H, Li M, Hasumi K, Guo WZ, Li XK (2021) Beta-glucan from Aureobasidium pullulans augments the anti-tumor immune responses through activated tumor-associated dendritic cells. Int Immunopharmacol 101(Pt A):108265. https://doi.org/10.1016/j.intimp.2021.108265
Geller A, Shrestha R, Yan J (2019) Yeast-derived beta-glucan in cancer: novel uses of a traditional therapeutic. Int J Mol Sci 20(15):3618. https://doi.org/10.3390/ijms20153618
Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH (2017) PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214(4):895–904. https://doi.org/10.1084/jem.20160801
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207(10):2187–2194. https://doi.org/10.1084/jem.20100643
Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, Guo J, Peng H, Chen M, Fu YX et al (2020) PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun 11(1):4835. https://doi.org/10.1038/s41467-020-18570-x
Shan T, Chen S, Chen X, Wu T, Yang Y, Li S, Ma J, Zhao J, Lin W, Li W et al (2020) M2TAM subsets altered by lactic acid promote Tcell apoptosis through the PDL1/PD1 pathway. Oncol Rep 44(5):1885–1894. https://doi.org/10.3892/or.2020.7767
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D et al (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545(7655):495–499. https://doi.org/10.1038/nature22396
Wei Y, Zhao Q, Gao Z, Lao XM, Lin WM, Chen DP, Mu M, Huang CX, Liu ZY, Li B et al (2019) The local immune landscape determines tumor PD-L1 heterogeneity and sensitivity to therapy. J Clin Invest 129(8):3347–3360. https://doi.org/10.1172/JCI127726
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K (2013) Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE 8(2):e57114. https://doi.org/10.1371/journal.pone.0057114
Zhu Z, Zhang H, Chen B, Liu X, Zhang S, Zong Z, Gao M (2020) PD-L1-mediated immunosuppression in glioblastoma is Associated with the infiltration and M2-polarization of tumor-associated macrophages. Front Immunol 11:588552. https://doi.org/10.3389/fimmu.2020.588552
Tsukihara H, Nakagawa F, Sakamoto K, Ishida K, Tanaka N, Okabe H, Uchida J, Matsuo K, Takechi T (2015) Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep 33(5):2135–2142. https://doi.org/10.3892/or.2015.3876
Z-j JIN (2004) About the evaluationof drug combination. Acta Pharmacol Sin 25(2):146–147
Wang K, Liu X, Liu Q, Ho IH, Wei X, Yin T, Zhan Y, Zhang W, Zhang W, Chen B et al (2020) Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis 11(8):611. https://doi.org/10.1038/s41419-020-02880-5
Shen N, Yang C, Zhang X, Tang Z, Chen X (2021) Cisplatin nanoparticles possess stronger anti-tumor synergy with PD1/PD-L1 inhibitors than the parental drug. Acta Biomater 135:543–555. https://doi.org/10.1016/j.actbio.2021.08.013
Charan J, Kantharia ND (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4(4):303–306. https://doi.org/10.4103/0976-500X.119726
Kang H (2021) Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof 18:17. https://doi.org/10.3352/jeehp.2021.18.17
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social. Behav Biomed Sci 39(2):175–191
Vetvicka V, Thornton BP, Ross GD (1996) Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest 98(1):50–61. https://doi.org/10.1172/JCI118777
Chitirala P, Chang HF, Martzloff P, Harenberg C, Ravichandran K, Abdulreda MH, Berggren PO, Krause E, Schirra C, Leinders-Zufall T, Benseler F, Brose N, Rettig J (2020) Studying the biology of cytotoxic T lymphocytes in vivo with a fluorescent granzyme B-mTFP knock-in mouse. Elife 9:e58065. https://doi.org/10.7554/eLife.58065
Packard BZ, Telford WG, Komoriya A, Henkart PA (2007) Granzyme B activity in target cells detects attack by cytotoxic lymphocytes. J Immunol 179(6):3812–3820. https://doi.org/10.4049/jimmunol.179.6.3812
Wattrang E, Dalgaard TS, Norup LR, Kjaerup RB, Lunden A, Juul-Madsen HR (2015) CD107a as a marker of activation in chicken cytotoxic T cells. J Immunol Methods 419:35–47. https://doi.org/10.1016/j.jim.2015.02.011
Anikeeva N, Panteleev S, Mazzanti NW, Terai M, Sato T, Sykulev Y (2021) Efficient killing of tumor cells by CAR-T cells requires greater number of engaged CARs than TCRs. J Biol Chem 297(3):101033. https://doi.org/10.1016/j.jbc.2021.101033
Frank B, Wei Y-L, Kim K-H, Guerrero A, Lebrec H, Balazs M, Wang X (2018) Development of a BiTE®-mediated CD8+ cytotoxic T-lymphocyte activity assay to assess immunomodulatory potential of drug candidates in Cynomolgus macaque. J Immunotoxicol 15(1):119–125. https://doi.org/10.1080/1547691X.2018.1486342
Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM (2008) Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 180(5):3132–3139. https://doi.org/10.4049/jimmunol.180.5.3132
Jiang T, Zhou C, Ren S (2016) Role of IL-2 in cancer immunotherapy. Oncoimmunology 5(6):e1163462. https://doi.org/10.1080/2162402X.2016.1163462
Gettinger SN, Choi J, Mani N, Sanmamed MF, Datar I, Sowell R, Du VY, Kaftan E, Goldberg S, Dong W et al (2018) A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat Commun 9(1):3196. https://doi.org/10.1038/s41467-018-05032-8
Soares A, Govender L, Hughes J, Mavakla W, de Kock M, Barnard C, Pienaar B, Janse van Rensburg E, Jacobs G, Khomba G et al (2010) Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods 362(1–2):43–50. https://doi.org/10.1016/j.jim.2010.08.007
Matsushita H, Hosoi A, Ueha S, Abe J, Fujieda N, Tomura M, Maekawa R, Matsushima K, Ohara O, Kakimi K (2015) Cytotoxic T lymphocytes block tumor growth both by lytic activity and IFNgamma-dependent cell-cycle arrest. Cancer Immunol Res 3(1):26–36. https://doi.org/10.1158/2326-6066.CIR-14-0098
Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H (2015) Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A 112(6):1809–1814. https://doi.org/10.1073/pnas.1417636112
Awad RM, De Vlaeminck Y, Maebe J, Goyvaerts C, Breckpot K (2018) Turn back the TIMe: targeting tumor infiltrating myeloid cells to revert cancer progression. Front Immunol 9:1977. https://doi.org/10.3389/fimmu.2018.01977
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M et al (2016) Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17(3):684–696. https://doi.org/10.1038/nnano.2016.168
Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M et al (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11(11):986–994
Kim J, Bae JS (2016) Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm 2016:6058147. https://doi.org/10.1155/2016/6058147
Di Mitri D, Toso A, Alimonti A (2015) Tumor-infiltrating myeloid cells drive senescence evasion and chemoresistance in tumors. Oncoimmunology 4(9):e988473. https://doi.org/10.4161/2162402X.2014.988473
Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD (2016) Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine 6:50–58
Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L (2013) CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 73(9):2782–2794. https://doi.org/10.1158/0008-5472.CAN-12-3981
Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM (2010) Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A 107(18):8363–8368
Cao Q, Huang Q, Wang YA, Li C (2021) Abraxane-induced bone marrow CD11b(+) myeloid cell depletion in tumor-bearing mice is visualized by muPET-CT with (64)Cu-labeled anti-CD11b and prevented by anti-CSF-1. Theranostics 11(7):3527–3539
de Graaff P, Govers C, Wichers HJ, Debets R (2018) Consumption of beta-glucans to spice up T cell treatment of tumors: a review. Expert Opin Biol Ther 18(10):1023–1040. https://doi.org/10.1080/14712598.2018.1523392
Kawata K, Iwai A, Muramatsu D, Aoki S, Uchiyama H, Okabe M, Hayakawa S, Takaoka A, Miyazaki T (2015) Stimulation of macrophages with the beta-glucan produced by aureobasidium pullulans promotes the secretion of tumor necrosis factor-related apoptosis inducing ligand (TRAIL). PLoS ONE 10(4):e0124809. https://doi.org/10.1371/journal.pone.0124809
Suzuki T, Kusano K, Kondo N, Nishikawa K, Kuge T, Ohno N (2021) Biological activity of high-purity β-1,3–1,6-Glucan derived from the black yeast aureobasidium pullulans: a literature review. Nutrients. https://doi.org/10.3390/nu13010242
Driscoll M, Hansen R, Ding C, Cramer DE, Yan J (2009) Therapeutic potential of various beta-glucan sources in conjunction with anti-tumor monoclonal antibody in cancer therapy. Cancer Biol Ther 8(3):218–225. https://doi.org/10.4161/cbt.8.3.7337
Kornete M, Sgouroudis E, Piccirillo CA (2012) ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol 188(3):1064–1074. https://doi.org/10.4049/jimmunol.1101303
Chen D, Liu X, Yang Y, Yang H, Lu P (2015) Systematic synergy modeling: understanding drug synergy from a systems biology perspective. BMC Syst Biol 9:56. https://doi.org/10.1186/s12918-015-0202-y
Duarte D, Vale N (2022) Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr Res Pharmacol Drug Discov 3:100110. https://doi.org/10.1016/j.crphar.2022.100110
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Lederer S, Dijkstra TMH, Heskes T (2018) Additive dose response models: explicit formulation and the loewe additivity consistency condition. Front Pharmacol 9:31. https://doi.org/10.3389/fphar.2018.00031
Liu Q, Yin X, Languino LR, Altieri DC (2018) Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat Biopharm Res 10(2):112–122. https://doi.org/10.1080/19466315.2018.1437071
Grosskopf AK, Correa S, Baillet J, Maikawa CL, Gale EC, Brown RA, Appel EA (2021) Consistent tumorigenesis with self-assembled hydrogels enables high-powered murine cancer studies. Commun Biol 4(1):985. https://doi.org/10.1038/s42003-021-02500-8
Fridman R, Benton G, Aranoutova I, Kleinman HK, Bonfil RD (2012) Increased initiation and growth of tumor cell lines, cancer stem cells and biopsy material in mice using basement membrane matrix protein (Cultrex or Matrigel) co-injection. Nat Protoc 7(6):1138–1144. https://doi.org/10.1038/nprot.2012.053
Fowlkes N, Clemons K, Rider PJ, Subramanian R, Wakamatsu N, Langohr I, Kousoulas KG (2019) Factors affecting growth kinetics and spontaneous metastasis in the B16F10 syngeneic murine melanoma model. Comp Med 69(1):48–54
Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, Logronio K, Tu GH, Tsaparikos K, Li X et al (2015) Combination of 4–1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res 3(2):149–160. https://doi.org/10.1158/2326-6066.CIR-14-0118
Grinshtein N, Ventresca M, Margl R, Bernard D, Yang TC, Millar JB, Hummel J, Beermann F, Wan Y, Bramson JL (2009) High-dose chemotherapy augments the efficacy of recombinant adenovirus vaccines and improves the therapeutic outcome. Cancer Gene Ther 16(4):338–350. https://doi.org/10.1038/cgt.2008.89
Baird JR, Byrne KT, Lizotte PH, Toraya-Brown S, Scarlett UK, Alexander MP, Sheen MR, Fox BA, Bzik DJ, Bosenberg M et al (2013) Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol 190(1):469–478. https://doi.org/10.4049/jimmunol.1201209
Reilley MJ, Morrow B, Ager CR, Liu A, Hong DS, Curran MA (2019) TLR9 activation cooperates with T cell checkpoint blockade to regress poorly immunogenic melanoma. J Immunother Cancer 7(1):323. https://doi.org/10.1186/s40425-019-0811-x
Bally AP, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, Boss JM (2015) NF-kappaB regulates PD-1 expression in macrophages. J Immunol 194(9):4545–4554. https://doi.org/10.4049/jimmunol.1402550
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX (2014) Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124(2):687–695. https://doi.org/10.1172/JCI67313
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al (2002) Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8):793–800. https://doi.org/10.1038/nm730
Acknowledgements
The authors are grateful to thank Ms. Y Sato for her invaluable technical assistance.
Funding
This study was supported by research grants from the Grants of Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid 18K08558, 21K15586, 21K08634); grants from the National Center for Child Health and Development (2021C-40, 29-09).
Author information
Authors and Affiliations
Contributions
XH, YFS, HH, MF, KK, WZG and XKL and conceived and designed the project; XH and YFS acquired the data; HH, MF, KK, WZG and XKL analyzed and interpreted the data; XH, YFS and XKL wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
KK is an employee of Aureo Co., Ltd.
Ethics approval
Mice were cared for in accordance with the National Research Institute for Child Health and Development (NCCHD) guidelines (A2017-005-C03) on laboratory animal welfare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, X., Shui, Y., Hirano, H. et al. PD-L1 antibody enhanced β-glucan antitumor effects via blockade of the immune checkpoints in a melanoma model. Cancer Immunol Immunother 72, 719–731 (2023). https://doi.org/10.1007/s00262-022-03276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03276-4