Abstract
Purpose
To assess indications, safety, and effectiveness of percutaneous adrenal mass biopsy in contemporary practice.
Methods
This institutional review board-approved, retrospective study included all patients undergoing percutaneous image-guided adrenal mass biopsies at an academic health system from January 6, 2015, to January 6, 2023. Patient demographics, biopsy indications, mass size, laboratory data, pathology results, and complications were recorded. Final diagnoses were based on pathology or ≥ 1 year of imaging follow-up when biopsy specimens did not yield malignant tissue. Test performance calculations excluded repeat biopsies. Continuous variables were compared with Student’s t test, dichotomous variables with chi-squared test.
Results
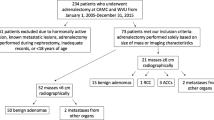
A total of 160 patients underwent 186 biopsies. Biopsies were indicated to diagnose metastatic disease (139/186; 74.7%), for oncologic research only (27/186; 14.5%), diagnose metastatic disease and oncologic research (15/186; 8%), and diagnose an incidental adrenal mass (5/186; 2.7%). Biopsy specimens were diagnostic in 154 patients (96.3%) and non-diagnostic in 6 (3.8%). Diagnostic biopsies yielded malignant tissue (n = 136), benign adrenal tissue (n = 12), and benign adrenal neoplasms (n = 6) with sensitivity = 98.6% (136/138), specificity = 100% (16/16), positive predictive value = 100% (136/136), and negative predictive value = 88.9% (16/18). Adverse events followed 11/186 procedures (5.9%) and most minor (7/11, 63.6%). The adverse event rate was similar whether tissue was obtained for clinical or research purposes (10/144; 6.9% vs. 1/42; 2.4%, p = 0.27), despite more specimens obtained for research (5.8 vs. 3.7, p < 0.001).
Conclusion
Percutaneous adrenal mass biopsy is safe, accurate, and utilized almost exclusively to diagnose metastatic disease or for oncologic research. The negative predictive value is high when diagnostic tissue samples are obtained. Obtaining specimens for research does not increase adverse event risk.
Graphical Abstract





Similar content being viewed by others
References
Lipnik AJ, Brown DB (2015) Image-Guided Percutaneous Abdominal Mass Biopsy: Technical and Clinical Considerations. Radiol Clin North Am 53:1049–1059
Mazzaglia PJ, Monchik JM. Limited value of adrenal biopsy in the evaluation of adrenal neoplasm: a decade of experience. Arch Surg. 2009 144(5):465-70
Krishna S, Moloney BM, Bao B, et al (2023) Adrenal Mass Biopsy in Patients Without Extra-Adrenal Primary Malignancy: A Multicenter Study. AJR Am J Roentgenol. https://doi.org/https://doi.org/10.2214/AJR.23.29826
Kahramangil B, Kose E, Remer EM, Reynolds JP, Stein R, Rini B, Siperstein A, Berber E (2022) A Modern Assessment of Cancer Risk in Adrenal Incidentalomas: Analysis of 2219 Patients. Ann Surg 275:e238–e244
Song JH, Chaudhry FS, Mayo-Smith WW (2008) The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 190:1163–1168
Fassnacht M, Tsagarakis S, Terzolo M, et al (2023) European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 189:G1–G42
Platzek I, Sieron D, Plodeck V, Borkowetz A, Laniado M, Hoffmann R-T (2019) Chemical shift imaging for evaluation of adrenal masses: a systematic review and meta-analysis. Eur Radiol 29:806–817
Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR (1998) Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 171:201–204
Zeiger MA, Thompson GB, Duh Q-Y, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J, American Association of Clinical Endocrinologists, American Association of Endocrine Surgeons (2009) The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract 15 Suppl 1:1–20
Rowe NE, Kumar RM, Schieda N, et al (2023) Canadian Urological Association guideline: Diagnosis, management, and followup of the incidentally discovered adrenal mass. Can Urol Assoc J 17:12–24
Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, Berland LL, Pandharipande PV (2017) Management of Incidental Adrenal Masses: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 14:1038–1044
Dalag L, Fergus JK, Zangan SM (2019) Lung and Abdominal Biopsies in the Age of Precision Medicine. Semin Intervent Radiol 36:255–263
Sone M, Sugawara S, Yatabe Y (2022) Role of Image-Guided Percutaneous Needle Biopsy in the Age of Precision Medicine. Curr Oncol Rep 24:1035–1044
Levit LA, Peppercorn JM, Tam AL, Marron JM, Mathews DJH, Levit K, Roach N, Ratain MJ (2019) Ethical Framework for Including Research Biopsies in Oncology Clinical Trials: American Society of Clinical Oncology Research Statement. JCO 37:2368–2377
Dermody SM, Shuman AG (2021) Implications of Research Biopsies in Clinical Trials. Oncologist 26:994–996
Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, Abbruzzese JL, Tam AL (2013) Use of Research Biopsies in Clinical Trials: Are Risks and Benefits Adequately Discussed? J Clin Oncol 31:17–22
Silverman SG, Mueller PR, Pinkney LP, Koenker RM, Seltzer SE (1993) Predictive value of image-guided adrenal biopsy: analysis of results of 101 biopsies. Radiology 187:715–718
Paulsen SD, Nghiem HV, Korobkin M, Caoili EM, Higgins EJ (2004) Changing role of imaging-guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol 182:1033–1037
Welch TJ, Sheedy PF, Stephens DH, Johnson CM, Swensen SJ (1994) Percutaneous adrenal biopsy: review of a 10-year experience. Radiology 193:341–344
Soosman SK, Schenker MP, Mazzola E, et al (2022) Safety of image guided research biopsies in patients with thoracic malignancies. Lung Cancer 173:53–57
Sharma KV, Venkatesan AM, Swerdlow D, DaSilva D, Beck A, Jain N, Wood BJ (2010) Image-Guided Adrenal and Renal Biopsy. Techniques in Vascular and Interventional Radiology 13:100–109
(2017) Common Terminology Criteria for Adverse Events (CTCAE)
Corwin MT, Navarro SM, Malik DG, Loehfelm TW, Fananapazir G, Wilson M, Campbell MJ (2019) Differences in Growth Rate on CT of Adrenal Adenomas and Malignant Adrenal Nodules. AJR Am J Roentgenol 213:632–636
Harisinghani MG, Maher MM, Hahn PF, Gervais DA, Jhaveri K, Varghese J, Mueller PR (2002) Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol 57:898–901
Bernardino ME, Walther MM, Phillips VM, Graham SD, Sewell CW, Gedgaudas-McClees K, Baumgartner BR, Torres WE, Erwin BC (1985) CT-guided adrenal biopsy: accuracy, safety, and indications. AJR Am J Roentgenol 144:67–69
Mody MK, Kazerooni EA, Korobkin M (1995) Percutaneous CT-guided biopsy of adrenal masses: immediate and delayed complications. J Comput Assist Tomogr 19:434–439
Bancos I, Tamhane S, Shah M, Delivanis DA, Alahdab F, Arlt W, Fassnacht M, Murad MH (2016) DIAGNOSIS OF ENDOCRINE DISEASE: The diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. Eur J Endocrinol 175:R65-80
Camacho A, Chung AD, Rigiroli F, Sari MA, Brook A, Siewert B, Ahmed M, Brook OR (2022) Concordance Assessment of Pathology Results with Imaging Findings after Image-Guided Biopsy. J Vasc Interv Radiol 33:159-168.e1
Yuan M, Huang L-L, Chen J-H, Wu J, Xu Q (2019) The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Sig Transduct Target Ther 4:1–14
Vanderveen KA, Thompson SM, Callstrom MR, Young WF, Grant CS, Farley DR, Richards ML, Thompson GB (2009) Biopsy of pheochromocytomas and paragangliomas: potential for disaster. Surgery 146:1158–1166
Zhang L, Åkerström T, Mollazadegan K, Beuschlein F, Pacak K, Skogseid B, Crona J (2023) Risk of complications after core needle biopsy in pheochromocytoma/paraganglioma. Endocr Relat Cancer 30:e220354
Acknowledgements
The authors wish to thank Laura E. Peterson for manuscript editing assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception/design of the work, participated in drafting and editing the manuscript, approved the final version, and are accountable for the manuscript contents.
Corresponding author
Ethics declarations
Ethical approval
This Health Insurance Portability and Accountability Act-compliant study received Institutional Review Board approval. None relevant to the current study. Dr. Glazer reports educational/travel support from Siemens Healthineers as well as compensation for expert testimony regarding abdominal radiology cases.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schenker, M.P., Silverman, S.G., Mayo-Smith, W.W. et al. Clinical indications, safety, and effectiveness of percutaneous image-guided adrenal mass biopsy: an 8-year retrospective analysis in 160 patients. Abdom Radiol 49, 1231–1240 (2024). https://doi.org/10.1007/s00261-024-04211-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-024-04211-0