Abstract
Cardiometabolic disease (CMD) encompasses a range of diseases such as hypertension, atherosclerosis, heart failure, obesity, and type 2 diabetes. Recent findings about CMD’s interaction with gut microbiota have broadened our understanding of how diet and nutrition drive microbes to influence CMD. However, the translation of basic research into the clinic has not been smooth, and dietary nutrition and probiotic supplementation have yet to show significant evidence of the therapeutic benefits of CMD. In addition, the published reviews do not suggest the core microbiota or metabolite classes that influence CMD, and systematically elucidate the causal relationship between host disease phenotypes-microbiome. The aim of this review is to highlight the complex interaction of the gut microbiota and their metabolites with CMD progression and to further centralize and conceptualize the mechanisms of action between microbial and host disease phenotypes. We also discuss the potential of targeting modulations of gut microbes and metabolites as new targets for prevention and treatment of CMD, including the use of emerging technologies such as fecal microbiota transplantation and nanomedicine.
Key points
• To highlight the complex interaction of the gut microbiota and their metabolites with CMD progression and to further centralize and conceptualize the mechanisms of action between microbial and host disease phenotypes.
• We also discuss the potential of targeting modulations of gut microbes and metabolites as new targets for prevention and treatment of CMD, including the use of emerging technologies such as FMT and nanomedicine.
• Our study provides insight into identification-specific microbiomes and metabolites involved in CMD, and microbial-host changes and physiological factors as disease phenotypes develop, which will help to map the microbiome individually and capture pathogenic mechanisms as a whole.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiometabolic disease (CMD), such as atherosclerosis, heart failure, hypertension, and hyperlipidemia (Tsao et al. 2022), is highly prevalent. The prevalence rate has been rising steadily for decades, becoming the main cause of death in the global population (Roth et al. 2020). Although the recommended treatment methods, including high-dose statins and lifestyle risk factors, were consistently used, the efficacy was not ideal, and adverse reactions still existed. The cause of CMD is still under study, but it is believed to be influenced by genetic and environmental factors. The risk factors associated with CMD can be unchangeable (genetic) or changeable (such as drinking, smoking, diet, and exercise). In conclusion, these observations indicated that many pathophysiological mechanisms of CMD had not been fully understood, and convincing data from clinical research, biochemistry, bioinformatics, pharmacology, and basic medicine would enhance our understanding of cardiovascular diseases (CMD) and the feasibility of prevention.
The human gastrointestinal tract is composed of trillions of microorganisms, including beneficial or pathogenic bacteria, fungi, and viruses. These microorganisms and their genomes are collectively referred to as intestinal flora (Loscalzo 2013). The intestinal flora and the metabolic function of the host interact, such as the decomposition and absorption of nutrients and the regulation of immune function, are essential to maintain the intestinal and external balance (Clemente et al. 2012; Loscalzo 2013). Recent studies have revealed the potential role of intestinal microbiome in CMD disease. And there is mounting evidence that changes in intestinal microbiota and its related metabolites may affect the initiation and progression of CMD, such as atherosclerosis, heart failure, and hypertension (Ascher and Reinhardt 2018; Koeth et al. 2013). The treatment of disease phenotype through fecal flora transplantation has been applied, which shows that the intestinal flora is sufficient to face the occurrence of CMD and atherosclerosis (Bäckhed et al. 2004; Ridaura et al. 2013), and also sufficient to transmit environmental protective role. The intestinal microbiome is also characterized as an endocrine organ that affects metabolism. However, this host-centric view largely ignores the complex nature of the intestinal microbiota.
In recent years, several study authors have summarized the role of gut microbiota in cardiometabolic disease (CMD) and the possible mechanisms by which probiotics intervene in cardiometabolic disease. These review articles provide theoretical basis for the intervention of CMD by targeting intestinal flora. However, these reviews did not suggest a core class of microbiota or metabolites that influence CMD, elucidate the causal relationship between host disease phenotypes-microbiome, or the possible application of personalized microbiome therapy for CMD. The aim of this review is to highlight the complex interaction of the gut microbiota and their metabolites with CMD progression and to further centralize and conceptualize the mechanisms of action between microbial and host disease phenotypes. We also discuss the potential of targeting modulations of gut microbes and metabolites as new targets for prevention and treatment of CMD, including the use of emerging technologies such as FMT and nanomedicine. This review is focused on three main key concepts. Firstly, there is increasing evidence that gut microbiota affects host physiological function through multiple pathways and specific receptors, and plays an important role in the pathology of host CMD. Thus, we describe the potential role of gut microbiota influencing CMD and the gut-X axis. Secondly, contributing roles of gut microbiota metabolites or secondary metabolites has been observed in attenuating CMD via neuroimmune pathways, as an immunomodulator or endocrine promoter. Thirdly, the rapid development of multi-omics techniques helps us to identify specific microbiomes and metabolites involved in CMD and to gain insight into microbial-host changes as disease phenotypes develop, which facilitates the development of live bacteria drugs and lead compounds, thus providing promising new avenues for therapeutic options. In this process, we identified knowledge gaps and limitations in current approaches to inform the development of drugs that regulate gut microbiota as new therapeutic targets for CMD.
Role of gut microbiota in host physiology
The human microbiome includes bacteria, viruses, single-celled eukaryotes, and archaea. And the microbes that live in symbiosis with the host are called microbiota, and their genome is called the “microbiome.” Lactobacillus, bifidobacteria, and other coliforms have been demonstrated as the most promising probiotics and their role in the prevention of degenerative diseases such as obesity, diabetes, cancer, cardiovascular disease, malignancies, liver disease, and inflammatory bowel disease (Azad et al. 2018). Intestinal microorganisms can also interact with elements of the host neuroendocrine system to modify behaviors relevant to social behavior, eating behavior, cognition, addiction, and sexual behavior (Cussotto et al. 2018). It is worth pointing out that gut microbes play an important role in mediating hormone release and thus regulating host metabolism. Gut microbes and metabolites influence the release of a variety of hormones by intestinal endocrine cells within the lining of the intestinal mucosa, including peptide YY, gastric inhibitory polypeptide, cholecystokinin, Glucagon-like peptide-1 (GLP-1), and 5-hydroxytryptamine (5-HT), which play a role in regulating key metabolic processes such as insulin sensitivity, fat storage, glucose tolerance, and appetite (Martin et al. 2019). In conclusion, gut microbiota can significantly affect host immunity and metabolism, and play an important role in maintaining the overall health of the host and resisting disease.
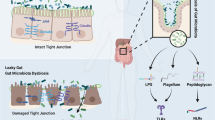
Intestinal leakage causes the entry of intestinal microbial derivatives and inflammation
Intestinal epithelial cells and the mucus they produce, as well as mucous membranes, form the intestinal barrier. The intestinal barrier prevents the invasion of pathogenic antigens. Impairment of intestinal wall and barrier function is often observed in patients with heart failure (Tang et al. 2019a). The increase of inflammatory factors in blood circulation is related to the severity of heart failure (Munger et al. 1996; Rauchhaus et al. 2000). Lipopolysaccharides produced by gram-negative bacteria in the gut can enter the host circulation through the impaired intestinal barrier. TLR (Toll-like receptor) on the surface of immune cells recognizes lipopolysaccharide and releases pro-inflammatory cytokines that put the host in a pro-inflammatory state (Hug et al. 2018). Some studies have shown that after Akkermania treatment, the level of lipopolysaccharide (LPS) in plasma of patients with metabolic syndrome (MS) is reduced (Depommier et al. 2019). Intestinal leakage is not the only cause of CMD. Many diseases are also associated with intestinal leakage. The relationship between CMD susceptibility and intestinal barrier damage changes in gut flora and metabolites, and host inflammatory response needs further exploration. Identifying the mechanism by which gut flora participates in the progress of CMD by triggering systemic immune response through the change of intestinal barrier will help to provide a new therapeutic approach.
Gut microbiome in CMD: altered composition of gut microbiota
An imbalance in the gut microbiome is called dysbiosis. The correlation between gut microbiota dysbiosis and many diseases/phenotypes has been a hot topic in the past decade. Identifying specific microbiomes associated with disease susceptibility is a fascinating research. Most gut microbial communities are composed of Bacteroides, Proteobacteria, Actinobacteria, Firmicutes, and Cerrucomicrobia, with Bacteroides and Firmicutes as the main phylum, and the ratio of Bacteroides to Firmicutes is also considered to be associated with the health (Tang et al. 2019b). However, there are differences among different individuals, different diseases, and different intestinal sites. The change of bacterial diversity is also associated with host genome and environmental factors. However, with the rapid development of sequencing technology, these microorganisms can be identified and characterized. It is worth mentioning that we need to identify a core group of bacteria and further study the pathogenesis of cardiovascular metabolic disorders from the perspective of bacterial functional genomics. Common diseases in which gut microbiota and their metabolites lead to CMD in the host through different pathways are shown in Fig. 1 (by Figdraw).
Hypertension
Hypertension is a risk factor on cardiovascular disease (Qato et al. 2018). The research in the experimental animal model showed that the regulation of gut flora was helpful to prevent hypertension (Jama et al. 2019b). Most studies have found that the gut microbiota of patients with hypertension was identified a reduced alpha diversity (De la Cuesta-Zuluaga et al. 2018; Jackson et al. 2018; Sun et al. 2019; Verhaar et al. 2020). Recent research by Yuan et al. also showed that Christensenellaceae and Streptococcaceae were the dominant microbes in the hypertensive animals (Yuan et al. 2020).
The absence of short-chain fatty acid (SCFA)–producing bacteria has been reported as a characteristic of gut flora in patients with hypertension (Calderon-Perez et al. 2020; Jama et al. 2019a). SCFAs can be activated by receptors to regulate renin secretion and achieve the purpose of BP regulation. The intestinal metabolite TMAO may also be related to the occurrence of hypertension. The specific mechanism may be related to more water reabsorption caused by aquaporin-2 (AQP-2) (Min et al. 2018). The possible pathways by which the gut microbiota may influence hypertension are illustrated in Fig. 2 (by Figdraw). A recent study has shown that the high-salt diet significantly changed the gut flora composition of rats, decreased bacteroidetes frailty and intestinal arachidonic acid levels, and increased the serum and intestinal corticosterone levels (Yan et al. 2020). Moreover, some aromatic amino acids (phenylalanine, tryptophan, tyrosine) metabolized in the intestines can also affect hypertension (** et al. 2021; Wang et al. 2021c; Yuan et al. 2021). A recent study showed that maternal 3, 3-dimethyl-1-butano could increase the plasma acetic acid, reduce the expression of GPR43 and GPR109A, and thus help to favor vasodilatation and lower BP (Hsu et al. 2021). SCFA in patients with acute myocardial infarction was significantly lower than that in healthy controls, indicating that it may be a marker for the diagnosis of transient myocardial infarction (Guo et al. 2021). Propionic acid has parasympathetic excitatory effect. Zhou et al. studied the nutritional intervention effect of propionic acid on the heart after myocardial infarction. The results showed that oral propionic acid might partially activate the parasympathetic nerve based on the gut-brain axis and improve ventricular electrical remodeling in a rat model with myocardial infarction (Zhou et al. 2021). However, a study of 441 adults living in the community found that the high concentration of SCFA in feces was negatively associated with the diversity of microbiota, and was associated with the markers of intestinal permeability, obesity, hypertension, and metabolic disorder. The authors believed that the association between high fecal SCFAs and obesity and heart metabolic disorder might be due to the low absorption efficiency of SCFA (De la Cuesta-Zuluaga et al. 2018). Yves A et al. have found that the absolute and relative increase of propionic acid in the cecum is associated with the alleviation of atherosclerosis, liver steatosis, and inflammation (Millet et al. 2022). After major intestinal cholesterol transporter Niemann-Pick C1-like protein 1 was inhibited by propionic acid, the levels of total cholesterol and low-density lipoprotein decreased in atherosclerosis model mice, attenuating atherosclerosis (Haghikia et al. 2022). Conversely, it is interesting that succinate may activate hypoxia-inducible factor 1-alpha, nuclear factor kappa beta, and renin angiotensin system to make vascular fibrosis and smooth muscle cell proliferation and migration; it can also bind GPR91 receptor to activate and mediate macrophage polarization and cause endothelial dysfunction (Hu et al. 2022a; Zhang et al. 2022). The study of Sarah K. Kirschner et al. showed that the plasma concentrations of propionate, isovalerate, and butyrate in patients with heart failure were significantly lower than those in healthy controls, while the concentrations of acetate and valerate were not different (Kirschner et al. 2022). SCFA also has effects on neuroimmunity and endocrine function. For example, when the concentration of propionic acid and butyric acid in the body of patients with congestive heart failure is high, heart function and leg muscles tend to be healthier (Dalile et al. 2019; Frampton et al. 2020). Furthermore, intestinal-derived SCFAs can activate its receptors GPR43 and GPR41 and control obesity by participating in fat accumulation and inhibiting lipolysis in adipocytes (Sun et al. 2018). The underlying mechanism between SCFAs and CMD is described in Fig. 4 (Alhabeeb et al. 2021; Chakaroun et al. 2023; Chen et al. 2020b; Hu et al. 2022b; Lu et al. 2022; Pakhomov and Baugh 2021; Yao et al. 2020; Yoo et al. 2022; Yukino-Iwashita et al. 2022) (by Figdraw).
The short-chain fatty acid (SCFA) is involved in the development of cardiometabolic disease (CMD) through multiple molecular mechanisms, such as anti-inflammation, lipid metabolism, and insulin sensitivity. RAAS, renin angiotensin aldosterone system; HDAC, histone deacetylase; GLP-1, Glucagon-like peptide-1; PYY, peptide YY
SCFA is usually regarded as the terminal product of microbial catabolism, representing the readout of saccharic metabolism of the entire microbial community, and plays an important role in various cardiovascular diseases. Hypertension, atherosclerosis, heart failure, and diabetes are all accompanied by changes in SCFA and SCFA-producing bacteria, which may be a new biomarker for risk prediction of CMD. The functions of SCFA in metabolism, neuroimmunity, and anti-inflammation play a certain role in cardiovascular homeostasis. Reducing the risk of cardiovascular disease by supplementing specific fiber diets or directly supplementing SCFA is considered promising. However, the role of single SCFAs or combined SCFAs in the pathophysiology of different cells and diseases needs further study.
TMAO
TMA produced from choline, betaine, and L-carnitine metabolized by intestinal flora becomes TMAO after oxidation. TMAO is an intestinal metabolite, and its potential role in CVD has been confirmed. TMAO level is associated with promoting atherosclerosis and a moderately increased risk of CVD. TMAO is also involved in such as platelet activation, endothelial dysfunction, and thrombosis (Zhu et al. 2020). TMAO can regulate the expression of SERPINE1 to promote blood coagulation, which can accelerate the formation of atherosclerosis (Díez-Ricote et al. 2022). Recent studies have shown that TMAO increased inflammatory factors such as intercellular adhesion molecule-1 (ICAM1) and interleukin-6 (IL-6), oxidized low-density lipoprotein, and induced CD36 expression, thus aggravating atherosclerosis, while MAPK/JNK inhibitors could reduce TMAO-induced CD36 expression (Catar et al. 2022; Geng et al. 2018; Huang et al. 2022a).
The level of TMAO in patients with heart failure is closely associated with adverse events (mortality and/or rehospitalisation) (Suzuki et al. 2019). Multiple population cohort prospective studies have shown that TMAO and its precursors such as carnitine are predictors of adverse cardiovascular events in patients with heart failure (Israr et al. 2021; Suzuki et al. 2019; Wei et al. 2022; Zhou et al. 2020). Furthermore, a large number of latest lines of evidence indicated that the high level of TMAO in blood circulation was also associated with the progression of hypertension and related CVD (Huang et al. 2022b; Li et al. 2022a; Yang et al. 2022). A study suggested that TMAO might be a target for berberine to improve hypertension and complications (Wang et al. 2022). Hypertension and circulating TMAO concentration demonstrated significant positive dose dependence (Ge et al. 2020). However, a study on the relationship between TMAO and hypertension showed that high total choline intake would increase the level of TMAO, and there was a significant negative correlation between total choline and BP within a certain range (He et al. 2022). The relationship between TMAO and hypertension has not been elucidated, and the involvement of TMAO in the pathophysiological process of hypertension needs to be further discovered. Another prospective cohort study (2088 Chinese community participants) showed that serum TMAO levels were positively associated with the risk of type 2 diabetes and elevated fasting blood glucose in middle-aged and elderly Chinese (Li et al. 2022d).
In addition, TMAO, a metabolite derived from gut microbiota, could induce cardiac hypertrophy and fibrosis, which involved Smad3 signal transduction (Li et al. 2019). A prospective population study by Tang et al. showed that in apparently healthy individuals, the risk of CVD can be predicted by the increase of plasma TMAO level (Tang et al. 2021). The above results indicated that inhibiting the production of TMAO by intestinal microorganisms could become a potential target for prevention and treatment of CVD. Vegetarian diet is considered to be associated with the decrease of TMAO in plasma (Smits et al. 2018). The association between TMAO and CMD has been corroborated. However, although TMAO is one of the markers of gut microbiome that has been thoroughly studied, its mechanism of action in the pathogenesis of CVD needs to be further verified and explored. Whether the precursor substances of TMAO can regulate or predict the occurrence of early CVD also needs further study. Finally, it is necessary to explore the specific symbiotic microflora related to the pathway of TMA transformation, so as to reconstruct healthier microflora with less TMAO in CVD patients through dietary methods.
BA
Bile is synthesized by the liver and consists mainly of BAs and cholesterol, which emulsifies and solubilizes lipids. Because BAs are toxic to bacterial cells, they selectively shape the microbiota profile of the human gut microbiota, which is recognized as an endocrine organ that plays a key role in host health. In addition, enzymes derived from gut bacteria can act on BAs, which may affect the composition of BA in the host intestine and circulation, thus affecting host physiology and metabolism (Molinero et al. 2019). Elucidating the mechanisms of microbiome-BAS-host interactions is a fascinating strategy for treating metabolic diseases.
The association between BA and cardiometabolic has been confirmed, but the causality between BA and cardiometabolic and the exact mechanism of the role of BA remain unclear. Meanwhile, there are significant differences in BA metabolism between humans and rodents, while most studies on BA and cardiometabolic diseases are conducted in rodents (Li and Dawson 2019). Through the determination of 136 young adults cardiometabolic risk factors and 8 kinds of plasma BA to reveal the relationship between BA level and cardiometabolic risk factors, the study showed that plasma BA level was associated with cardiometabolic and inflammatory risk factors (Osuna-Prieto et al. 2022). BA is believed to activate FXR receptor, vitamin D receptor, pregnane X receptor, and G protein–coupled receptor Gpbar1 to affect cardiometabolic diseases (Li et al. 2020a; Wang et al. 2016; Yang et al. 2021e). However, the complex relationship between BA metabolism, lipid metabolism, and FXR signal remains to be further studied (Callender et al. 2022; Wang et al. 2018a).
Although the mechanism of BA regulating CVD in cardiovascular tissues remains unclear, circulating BA levels can predict the occurrence of coronary artery disease (Chong Nguyen et al. 2021). Intriguingly, there is an association between BA signal transduction and salt-sensitive hypertension. BA may have the functions of regulating inflammation and balancing body fluids in patients with salt-sensitive hypertension. FXR and TGR5 are considered potential therapeutic targets. There is evidence that INT-767, as an agonist of FXR and TGR5, can effectively attenuate obesity and atherosclerosis in animal models (Ishimwe et al. 2022; Jadhav et al. 2018). Naringin has been proved to play an anti-atherosclerotic role by regulating BA synthesis through FXR/FGF15 signaling pathway (Wang et al. 2021b). However, intriguingly, intestinal FXR has also been proved to regulate the transcriptional activity of SMPD3 to promote the production of ceramide, thereby increasing the instability of atherosclerotic plaque (Wu et al. 2021). A study of sterol sulfate significantly improved atherosclerotic in high-fat-diet-fed Apo E2/2 mice showed changes in BA profiles in the liver, gallbladder, serum, and stool, and increased FXR expression in the liver, suggesting that FXR was one of the pathways through which BAs were involved in sterol sulfate in attenuating atherosclerosis (Ding et al. 2021). Therefore, BA and BA receptors play an important role in atherosclerosis, mediating the regulation of signaling pathways including inflammation, oxidative stress, and lipoprotein (Qi et al. 2022). Meanwhile, a prospective cohort study by Mayerhofer et al. showed a decrease in BA levels in patients with chronic HF (Mayerhofer et al. 2017). In addition, BA level also affects glucose metabolism and T2DM (Zhang et al. 2019a). Activation of TGR5, one of the important receptors of BA, has been shown to be beneficial for improving T2DM (Du et al. 2022), with significantly reduced blood glucose levels in T2DM patients treated with high-dose TGR5 agonist sdb-756050 (Hodge and Nunez 2016). However, activation of FXR is also considered to be beneficial to improving T2D (Du et al. 2022). The role of gut flora can synthesize different BAs. The specific role of signal molecules generated by the interaction between BA and intestinal flora and the role of TGR5 and FXR receptors signal in maintaining glucose homeostasis remains to be further confirmed (Gao et al. 2022).
It is significantly important to maintain the homeostasis balance between gut flora and BAs to play physiological functions in various metabolic pathways and prevent pathological progression of diseases. The complex association between BA metabolism, FXR signaling, and dyslipidemia may be a new target for intervention in CMD (Callender et al. 2022), but nevertheless, the causal mechanism between changes in BA signaling and CMD needs to be further investigated.
Others
A series of metabolites produced by gut flora play a crucial role in human physiology, among which the three main tryptophan (Trp) metabolic pathways mediated by intestinal flora such as serotonin (5-hydroxytryptophan), kynine (Kyn), and indole derivatives have received extensive attention. Allison et al. also summarized the main ways that tryptophan, a metabolite of intestinal flora, affects diseases, especially the 5-HT, Kyn, and AhR pathways, which are affected differently in diseases, but are still closely connected (Agus et al. 2018).
In addition to tryptophan, phenylalanine and its metabolized tyrosine are also aromatic amino acids that can affect neural immunity. Tyrosine can be further converted into neurotransmitters, adrenaline, and noradrenaline (** et al. 2021). In patients with advanced atherosclerosis, tryptophan, indole-3-propionic acid, hippuric acid, and other phenyl-derived metabolites have changed and are associated with atherosclerosis (Cason et al. 2018). Our previous study found that L-phenylalanine, L-valine, and L-methionine were three metabolites involved in intestinal and blood metabolism in L-NAME-induced hypertensive rats, which may be related to the development mechanism of hypertension (Yuan et al. 2022). Studies have shown that phenylalanine can be metabolized by gut microbiota to produce phenylpyruvic acid (Dodd et al. 2017; Nemet et al. 2020) and finally produce polyacrylic acid. Polyacrylic acid is then circulated through the portal venous system to be metabolized in the liver to produce Phenylacetylglutamine (PAGln) (Nemet et al. 2020). A recent study also found that the key bacterial enzyme porA for PAGln synthesis increased in patients with atrial fibrillation. PAGln might participate in the pathogenesis of atrial fibrillation by promoting oxidative stress and apoptosis of atrial muscle cells (Fang et al. 2022).
Furthermore, a study has shown elevated PAGln level is an independent risk factor for HF and is associated with a higher risk of cardiovascular death (Zong et al. 2022). Meanwhile, elevated PAGln levels were also associated with acute myocardial infarction and T2DM (Tang et al. 2022). However, as a potential biomarker of cardiovascular disease, the mechanism by which PAGln acts through gut-heart needs to be verified by more cohort studies (Chen et al. 2020a).
Potential therapeutic means
Metabolites produced by gut flora through fermentation of indigestible fibers can be partially absorbed into the host circulation, thus acting on remote target organs. Metabolites produced by microorganisms may have synergistic effects on promoting human health. The relationship between host CMD susceptibility and changes in microbiota and metabolites makes intestinal microbiota a new target for CMD therapy. The possible directions of application of targeting the gut microbiota to treat CMD are illustrated in Fig. 5 (by Figdraw).
Repair the impaired intestinal barrier and reduce inflammation
The apical junctional complex consisting of mucus and tight junctions between intestinal epithelial cells and luminal contents together make up the intestinal barrier (Lewis and Taylor 2020; Yang et al. 2021c). Commensal bacteria are present in the mucus layer of the intestinal lumen. As a result of mucin cleavage, the loose mucus expands in volume, allowing bacteria to penetrate the mucus. Gut microbiota and their metabolites can affect the host’s neuroimmune regulation after entering the circulation system through intestinal leakage (Yousefi et al. 2022). After the occurrence of intestinal leakage, the thickness of mucus in the luminal decreases, leading to the entry of intestinal bacteria and antigens into the circulation, thereby triggering systemic inflammatory pathways (Feng et al. 2019; Ma and Li 2018), which increases the risk of cardiovascular diseases (CVD) (Manolis et al. 2022). Adherence to the Mediterranean diet is associated with fewer adverse cardiovascular events and lower circulating LPS levels (Pastori et al. 2017). Dietary bioactive molecules synthesized and metabolized by gut microbes can affect hypertension after entering the circulation system (Cookson 2021).
Brandsma et al. believed that identified pattern recognition receptors and antimicrobial peptides as key factors controlling the composition of the gut microbiota, and defects in these genes lead to leakage of endotoxin into the circulation, resulting in low-grade chronic inflammation and insulin resistance (Brandsma et al. 2015). T2D metabolic inflammation is also known to be associated with increased intestinal permeability and gut-derived lipopolysaccharide translocation. A number of other natural foods have also been proposed to repair the intestinal barrier, reduce intestinal inflammation, and inhibit intestinal metabolic disorders (Hu et al. 2021; Yang et al. 2021d; Yuan et al. 2021). For example, Berberine, which improves intestinal barrier function and reduces inflammation caused by intestinal microbiota-derived LPS, is considered to have the potential to treat atherosclerotic cardiovascular disease and metabolic diseases (Yang et al. 2021c). In conclusion, prevention of the occurrence of inflammation, increased intestinal permeability, and damaged intestinal barrier caused by intestinal leakage may be potential means to intervene in CMD.
Targeted regulation of gut microbiota
Dietary patterns can regulate gut microbiota to prevent CMD
The gut flora catabolisms the unused dietary components of the host, which is associated with the multiple metabolisms of central carbon and amino acids and the biosynthesis of secondary metabolites (Griffin et al. 2015; Xu and Yang 2021). The metabonomic evidence showed that the metabolites of gut flora affected the host physiology (Xu and Yang 2020), and regulated the level of these metabolites in the circulation may help prevent the occurrence of CMD (Griffin et al. 2015; Xu and Yang 2020). Dietary polysaccharides, flavonoids, polyphenols, and other compounds can regulate gut microbiota and affect the production of gut microbiota–derived metabolites (Feng et al. 2022), and constraint-based modeling analysis is considered to be a promising method to study the interaction between diet and gut microbiota (Blasco et al. 2021).
A large body of research have shown that dietary polyphenols have beneficial effects in improving hypertension and hyperlipidemia and promoting the regeneration of vascular endothelial cells (Szczepańska et al. 2022). In addition, coenzyme Q10, which is rich in foods such as soybeans and nuts, has been proved to have the function of protecting the heart and blood vessels, and the mechanisms by which play a cardioprotective role include increasing ATP production in myocardial cells, antioxidation, improving fat content and improving endothelial function (Gutierrez-Mariscal et al. 2021; Testai et al. 2021). Sterols from plant diet, such as sitosterol, have the effect of antioxidation and improving blood lipids (Babu and Jayaraman 2020; Szczepańska et al. 2022). Other food components beneficial to CMD include Omega-3 and Vitamin E, which can reduce platelet aggregation and reduce the risk of CVD (Cao and Weaver 2022; Innes and Calder 2020; Margina et al. 2020).
The previous studies argued that the dietary patterns that were conducive to CMD and lowered the risk of death from CVD include Mediterranean diet (Martínez-González et al. 2019; Rees et al. 2019; Salas-Salvadó et al. 2018), the DASH diet (Chiavaroli et al. 2019; Filippou et al. 2020; Juraschek et al. 2020; Liu et al. 2021a), and Vegetarian diets (Chiu et al. 2020; Quek et al. 2021; Trautwein and McKay 2020). As in the Wang et al. study, adherence to the Mediterranean diet would have a significant impact on the gut microbiota related to SCFA production, secondary BA production and degradation of plant-derived polysaccharides, and the lack of P. cpori in the gut microbiota of a group of participants, which may be the reason for their risk protection of heart metabolic diseases (Wang et al. 2021a). Due to the heterogeneity of individuals, different dietary patterns will affect diet-related individual metabolic patterns; however, the relationship between individual metabolic characteristics and CMD has been confirmed. The characteristics of dietary-derived metabolites are generally important in CMD. Ravi V. Shah et al. found that food components/catabolites (such as fish and long-chain unsaturated triacylglycerol) interacted with host characteristics (e.g., intestinal flora) and involved in CMD- and CVD- related pathways (e.g., ceramide/sphingomyelin lipid metabolism) (Shah et al. 2022). Therefore, more population cohorts need to be designed, and personalized diet should be designed based on individual metabolic characteristics.
Probiotics
Probiotics, known as the next generation of viable bacteriological drugs, change the composition and metabolites of gut flora through colonization in the gut, which is closely related to human health, especially the protective effect on cardiometabolic diseases such as hypertension, atherosclerosis, and insulin resistance (Chi et al. 2020; Leustean et al. 2018; O'Morain and Ramji 2020). In recent years, a large body of researches have accumulated in vivo experimental data of probiotics to lower BP. Several meta-analysis studies have shown that probiotics may lower BP, which is expected to provide a new method for the treatment of hypertension (Chi et al. 2020; Lewis-Mikhael et al. 2020; Liang et al.; Qi et al. 2020). In a meta-analysis of data from 11 randomized controlled trials (RCTs), probiotic supplementation lowered BP in patients with T2DM (Hendijani and Akbari 2018). However, the data were not entirely consistent; Dong et al.’s meta-analysis of 18 trials in patients with MS showed no significant changes in BP caused by probiotic supplementation (Dong et al. 2019). The research of probiotic intervention in diabetes is also one of the ongoing hot topics. A meta-analysis of 13 RCTs found that supplementation with probiotics reduced FBG levels but did not change HbA1c levels (Ardeshirlarijani et al. 2019). The study of Liang et al. (2020) was similar, especially the mixed probiotics had a great effect on the level of FBG but had no effect on the level of HbA1c and insulin (Liang et al.). However, two meta-analyses showed that probiotics did not have any significant effects on markers of glucose metabolism and insulin function (Barengolts et al. 2019; Dong et al. 2019). Multiple meta-analyses evaluating the potential of probiotics to regulate lipid levels have shown that probiotic supplements help improve dyslipidemia (Companys et al. 2020; Hadi et al. 2020; Hendijani and Akbari 2018; Liang et al.; Yan et al. 2019). In addition, probiotics also regulate metabolites in the gut that are associated with cardiovascular disease. Liang et al. used Lactis F1-3–2 intervention to regulate TMA-TMAO in cecum and serum, improve lipid metabolism, and attenuate TMAO-induced atherosclerosis (Liang et al. 2020). In conclusion, probiotics have the effect of regulating blood glucose, BP, and blood lipids, which is beneficial to improve CMD such as hypertension, diabetes, and atherosclerosis. However, other studies have shown that probiotics supplementation did not significantly improve the levels of CMD-related markers. Therefore, further population-based studies with larger cohorts are needed to verify the effect of probiotics on improving CMD by removing the influencing factors of other diseases except CMD. Nevertheless, probiotics still show great potential in the intervention of CMD. Moreover, the evaluation of the safety and genetic stability of probiotics is also significantly important. After clinical and evidence-based medical evaluation, probiotics are expected to become oral microbial drugs in vivo.
Improve the metabolism of gut microbiota
Dietary intervention regulates SCFAs
It is of particular importance to improve chronic metabolic diseases by regulating intestinal microbial function and metabolites through dietary intervention (Wu et al. 2011). More fiber intake is believed to produce more SCFAs. Animal-based diet and plant-based diet would affect the production of gut flora and gut flora metabolite SCFAs. Animal-based diet could significantly reduce the concentration of acetate and butyrate in the feces of the host (David et al. 2014).
Numerous studies have implicated the association between gut flora, diet, and health, and different diets can affect human health and CMD risk. The Mediterranean diet, for example, emphasizes a higher intake of vegetable and grains, which is associated with higher SCFA levels and gut flora richness (Garcia-Mantrana et al. 2018). Dietary fiber promoted the increase of SCFA-producing strains, increased species diversity and richness, improved glycosylated hemoglobin, and decreased production of harmful compounds such as indoles and hydrogen sulfide in T2DM patients (Zhao et al. 2018). The diet with low dietary fiber would not only reduce the production of SCFA, but also increased the production of metabolites harmful to mucin. A study showed that dietary fiber deprivation might be the cause of damage to the colonic mucus barrier (Desai et al. 2016). Low prebiotic fiber diet may lead to CMD. David et al. showed that the possible causes of hypertension due to the deficiency of prebiotic fibers include the formation of hypertensinogenic intestinal flora and the reduction of SCFA production (Kaye et al. 2020).
Intervention with CMD based on microbial SCFA diet may have good effect, high safety, and high compliance. However, due to the huge heterogeneity of individual gut flora, the metabolites produced by it may act on multiple tissues and targets (Turnbaugh 2020). Therefore, it is necessary to establish personalized diet strategies and conduct more population experiments to verify its effects.
Dietary and pharmacological interventions targeting TMAO levels
Diets rich in TMAO precursors, such as red meat and eggs, and consumption of animal protein and saturated fats may increase the amount of circulating TMAO. The intake of choline and carnitine in the diet can affect the level of circulating TMAO. Compared with omnivores, vegetarians have lower levels of circulating TMAO (Koeth et al. 2013). However, a predominantly red meat–based diet would increase the concentration of TMAO in the circulation, and changing the red meat–based diet to a white meat non meat protein diet could reduce the circulating TMAO concentration in a few weeks (Wang et al. 2019). The increase of circulating TMAO level by high-fat diet may be associated with the increase of oxygen and nitrate utilization after damaging the oxygen uptake of mitochondria to host intestinal epithelial cells (Yoo et al. 2021).
Therefore, it is possible to reduce the intake of TMAO from foods in the diet, such as choosing plant proteins as sources of protein or supplementing the Mediterranean diet, to promote cardiovascular health and reduce the incidence of cardiovascular events (Estruch et al. 2018; Mei et al. 2021; Yang et al. 2021b). It is an urgent problem to develop an economical and effective diet to reduce TMAO and attenuate CMD, which has potential significant public health benefits, among which plant-based substances also deserve attention (Iglesias-Carres et al. 2021).
Other techniques targeting gut microbiota to improve CMD
FMT
FMT has received widespread attention worldwide as an ecological therapy to restore the patient’s gut microbiota. A large body of preclinical and clinical studies have shown that FWT can be used in the treatment of CMD (Zheng et al. 2022). FMT can be used for the treatment of T1D and T2D. Studies have shown that β cell function is restored in T1D patients after FMT (Zhang et al. 2019b) Another study also showed that patients with T2D could maintain normal metabolism after diet-induced weight loss after autologous FMT (Chang et al. 2015). Previous research by our team has found that Washed Microbiota Transplantation (WMT) could also lower BP in patients with hypertension, especially in those who did not take antihypertensive drugs (Zhong et al. 2021). Moreover, a retrospective analysis of the improvement of blood glucose after WMT treatment for various reasons in the First Hospital of Guangzhou Pharmaceutical University from December 2016 to April 2022 found that WMT also significantly improved FBG (Wu et al. 2022a). Interestingly, the recent study from our group, which included 237 patients (42 with MS and 195 without MS), found that WMT treatment significantly restored gut microbiota homeostasis and improved blood glucose, lipid profile, BP, and BMI in patients with MS (Wu et al. 2022b). Apart from that, FMT is also making continuous attempts and breakthroughs in the treatment of tumor, nerve, and autoimmune diseases (Opoku-Acheampong et al. 2022). Most studies have shown that the transplantation of microorganisms into the gut of patients with CMD results in changes in the disease phenotype. The microbiota of the disease donor would replicate in the mouse gut. However, there were also cases in which FMT failed to develop a pathological phenotype in the recipient animals (Walter et al. 2020). In addition, probiotics that have been evaluated by clinical and evidence-based medicine may be added to the donor stool for personalized reinforcement of the donor microbiota based on patient characteristics. FMT has been fully recognized for the treatment of diseases, especially digestive tract diseases, but its role in the treatment of CMD is still controversial. In particular, the application of FMT is limited by the selection of donors and the heterogeneity of donor feces (Zheng et al. 2022). More clinical trial data are needed to promote the progress of FMT technology. It is necessary to optimize the design and develop more standardized application guidelines in the future.
Microbiota and nanomedicine-based approaches
Nanomedicine has been widely used in the diagnosis and targeted therapy of cancer, CMD, and other diseases, especially in the delivery of drugs as a carrier and the improvement of pharmacokinetics. Nanotechnology can promote the delivery of microbes and their lead compounds for targeted therapy of gut microbiota, protein profiling, and cholesterol modulation such as clearance of low-density lipoprotein cholesterol, thus promoting the prevention and treatment of CMD (Kazemian et al. 2020). Nanotechnology can be applied to modulate immunity and alleviate inflammation (Gan et al. 2018). Wang et al. applied broad-spectrum ROS-scavenging nanoparticles to reduce oxidative stress and thereby alleviate atherosclerosis (Wang et al. 2018b). Nanotechnology based on extracellular vesicles has brought opportunities for the development of nanomedicine. Exosomes and outer membrane vesicles regulate intestinal immunity and homeostasis by participating in the interaction between intestinal epithelial cells, immune cells, and gut flora (Li et al. 2022b). Intestinal bacteria and metabolites can change the physicochemical properties of nanoparticles, and nanoparticles can regulate bacterial metabolism and communication with intestinal lymphoid tissues (Javed et al. 2020). Based on the interaction between nanoparticles and intestinal flora, and the application of nanotechnology in the delivery and release of probiotics, it has the potential to apply it to target gut flora for the treatment of CMD. However, more biological studies are needed regarding the clinical application of nanotechnology-based particulate products (Mahmoudi 2018). As for nanomaterial as an emerging interdisciplinary discipline offers fascinating prospects for the treatment of CMD. However, the association between gut flora, nanomedicine, and CMD needs more clinical and cohort studies.
Conclusions and future perspectives
The gut microbiome profile of CMD has been initially described and is characterized by decreased gut microbiota abundance, absence of SCFA-producing bacteria, and abnormal trimethylamine metabolism, which are more closely related to metabolic disorders. A precise description and correlation study of CMD phenotype and intestinal physiological function were carried out, and combined with causal studies. Personalized microbiome therapy may be the most promising direction, which may require targeted capture of functional strains or genes that affect the progression of CMD and the physiological function of the host gut. The association of gut microbiota changes with distal target organs is also of interest, including the gut-heart, gut-vascular, gut-liver, and gut-brain axes. In addition, enterovirus studies, especially bacteriophages, have also been considered to have the potential to target the regulation of the gut microbiome due to their specificity (Hsu et al. 2019; Shkoporov and Hill 2019). Combined enterovirus and whole genome sequencing results from 196 patients with MS (a highly prevalent clinical condition preceding cardiometabolic disease) showed that the enterovirus was associated with a highly widely distributed intestinal phage family, named Candidatus Heliusviridae (de Jonge et al. 2022).
Challenges remain in fully integrating gut microbiota into clinical design. The variation factors of gut flora are very complex. The results of increased opportunistic pathogens in the gut of CMD patients are not completely reproducible, which may be related to the large individual differences. There is also no uniform standard for fecal sample collection for gut microbiota research, and different intestinal segments and different collection methods will contain different microbiota. In order to solve the above problems, it is necessary to turn the correlation study to the causal mechanism study, explore the exact downstream molecular pathway, carry out a large-scale prospective cohort study based on the population, and clinical and rodent correlation study to reveal the complex mechanism between gut flora and CMD. However, the factors driving causality may be diverse, including the host intestinal environment, intestinal transit time, and PH changes. In addition, we can also consider the method of gene editing to tap fluorescent proteins into bacteria or find specific molecular targets of bacteria for visual tracking, which is helpful to explore the pathway and target of the role of bacteria.
The small intestine plays a key role in digestion and nutrient absorption, and bacteria have a great impact on metabolism, intestinal barrier, immune response, etc. However, the overall stable changes of intestinal flora caused by CMD phenotype may also occur at time intervals, and the changes in different intestinal segments may not be consistent. Therefore, we propose that nanotechnology may be a method for gut microbiome research. Although the causal mechanism between gut microbiome and CMD is still unclear, there has been substantial evidence that the changes of gut microbiota may be the cause of CMD; in particular, the application of germ-free mice and FMT have brought us numerous animal research and clinical research data. In order to fully elucidate the specific mechanism of intestinal microbiome mediating CMD, and to guide the therapeutic intervention of CMD via probiotics, precision nutrition targeting intestinal metabolites, FMT, and advanced nanomedicine technology, thus we must elucidate the physiological factors that jointly affect the microbiome and disease, delineate the personalized microbiome profile, and capture a holistic view of pathogenic mechanisms.
Data Availability
The authors declare that all of the data and the material used in this study are available within this article. All data generated or analyzed in this study can be obtained from the authors upon reasonable request.
References
Agus A, Planchais J, Sokol H (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23(6):716–724
Alhabeeb H, AlFaiz A, Kutbi E, AlShahrani D, Alsuhail A, AlRajhi S, Alotaibi N, Alotaibi K, AlAmri S, Alghamdi S, AlJohani N (2021) Gut hormones in health and obesity: the upcoming role of short chain fatty acids. Nutrients 13(2) https://doi.org/10.3390/nu13020481
Alvarez-Silva C, Kashani A, Hansen TH, Pinna NK, Anjana RM, Dutta A, Saxena S, Støy J, Kampmann U, Nielsen T (2021) Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India. Genome Med 13(1):1–13
Arany Z, Neinast M (2018) Branched chain amino acids in metabolic disease. Curr Diabetes Rep 18(10):1–8
Ardeshirlarijani E, Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar Jalili R (2019) Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. Daru 27(2):827–837
Ascher S, Reinhardt C (2018) The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur J Immunol 48(4):564–575
Aydin O, Nieuwdorp M, Gerdes V (2018) The gut microbiome as a target for the treatment of type 2 diabetes. Curr Diabetes Rep 18(8) https://doi.org/10.1007/s11892-018-1020-6
Azad M, Kalam A, Sarker M, Li T, Yin J (2018) Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int 2018(undefined):9478630. https://doi.org/10.1155/2018/9478630
Babu S, Jayaraman S (2020) An update on beta-sitosterol: a potential herbal nutraceutical for diabetic management. Biomed Pharmacother 131 https://doi.org/10.1016/j.biopha.2020.110702
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci 101(44):15718–15723
Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T (2019) The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: a meta-analysis of nine randomized controlled trials. Nutrients 11(3):671
Belda E, Voland L, Tremaroli V, Falony G, Adriouch S, Assmann KE, Prifti E, Aron-Wisnewsky J, Debédat J, Le Roy T (2022) Impairment of gut microbial biotin metabolism and host biotin status in severe obesity: effect of biotin and prebiotic supplementation on improved metabolism. Gut 71:2463–2480
Blasco T, Pérez-Burillo S, Balzerani F, Hinojosa-Nogueira D, Lerma-Aguilera A, Pastoriza S, Cendoya X, Rubio Á, Gosalbes M, Jiménez-Hernández N, Pilar Francino M, Apaolaza I, Rufián-Henares J, Planes F (2021) An extended reconstruction of human gut microbiota metabolism of dietary compounds. Nat Commun 12(1):4728. https://doi.org/10.1038/s41467-021-25056-x
Bose S, Ramesh V, Locasale JW (2019) Acetate metabolism in physiology, cancer, and beyond. Trends Cell Biol 29(9):695–703
Brandsma E, Houben T, Fu J, Shiri-Sverdlov R, Hofker MH (2015) The immunity-diet-microbiota axis in the development of metabolic syndrome. Curr Opin Lipidol 26(2):73–81. https://doi.org/10.1097/mol.0000000000000154
Calderon-Perez L, Gosalbes MJ, Yuste S, Valls RM, Pedret A, Llaurado E, Jimenez-Hernandez N, Artacho A, Pla-Paga L, Companys J, Ludwig I, Romero M-P, Rubio L, Sola R (2020) Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep-UK 10(1) https://doi.org/10.1038/s41598-020-63475-w
Callender C, Attaye I, Nieuwdorp M (2022) The interaction between the gut microbiome and bile acids in cardiometabolic diseases. Metabolites 12(1):65
Cao S, Weaver CM (2022) Bioactives in the food supply: effects on CVD health. Curr Atheroscler Rep 24(8):655–661. https://doi.org/10.1007/s11883-022-01040-8
Cason CA, Dolan KT, Sharma G, Tao M, Kulkarni R, Helenowski IB, Doane BM, Avram MJ, McDermott MM, Chang EB (2018) Plasma microbiome-modulated indole-and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg 68(5):1552-1562.e7
Catar R, Chen L, Zhao H, Wu D, Kamhieh-Milz J, Luecht C, Zickler D, Krug AW, Ziegler CG, Morawietz H, Witowski J (2022) Native and oxidized low-density lipoproteins increase the expression of the LDL receptor and the LOX-1 receptor, respectively, in arterial endothelial cells. Cells 11(2) https://doi.org/10.3390/cells11020204
Caussy C, Loomba R (2018) Gut microbiome, microbial metabolites and the development of NAFLD. Nat Rev Gastro Hepat 15(12):719–720
Chakaroun RM, Olsson LM, Backhed F (2022) The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat Rev Cardiol. https://doi.org/10.1038/s41569-022-00771-0
Chakaroun RM, Olsson LM, Backhed F (2023) The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat Rev Cardiol 20(4):217–235. https://doi.org/10.1038/s41569-022-00771-0
Chang C-J, Lin C-S, Lu C-C, Martel J, Ko Y-F, Ojcius DM, Tseng S-F, Wu T-R, Chen Y-YM, Young JD (2015) Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun 6(1):1–19
Chen W, Zhang S, Wu J, Ye T, Wang S, Wang P, **ng D (2020a) Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin Chim Acta 507:236–241
Chen X-F, Chen X, Tang X (2020b) Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci 134(6):657–676. https://doi.org/10.1042/cs20200128
Chi C, Li C, Wu D, Buys N, Wang W, Fan H, Sun J (2020) Effects of probiotics on patients with hypertension: a systematic review and meta-analysis. Curr Hypertens Rep 22(5):1–8
Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, Salas-Salvadó J, Kendall CW, Sievenpiper JL (2019) DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients 11(2):338
Chiu TH, Chang H-R, Wang L-Y, Chang C-C, Lin M-N, Lin C-L (2020) Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 94(11):e1112–e1121
Chong Nguyen C, Duboc D, Rainteau D, Sokol H, Humbert L, Seksik P, Bellino A, Abdoul H, Bouazza N, Treluyer J-M (2021) Circulating bile acids concentration is predictive of coronary artery disease in human. Sci Rep-UK 11(1):1–10
Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270
Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, Valls RM (2020) Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: a systematic review and meta-analysis. Adv Nutr 11(4):834–863
Cookson TA (2021) Bacterial-induced blood pressure reduction: mechanisms for the treatment of hypertension via the gut. Front Cardiovasc Med 8:721393
Cornejo-Pareja I, Munoz-Garach A, Clemente-Postigo M, Tinahones FJ (2019) Importance of gut microbiota in obesity. Eur J Clin Nutr 72(1):26–37
Cussotto S, Sandhu KV, Dinan TG, Cryan JF (2018) The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrin 51:80–101
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K (2019) The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastro Hepat 16(8):461–478. https://doi.org/10.1038/s41575-019-0157-3
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559–563
de Jonge PA, Wortelboer K, Scheithauer TPM, van den Born BJH, Zwinderman AH, Nobrega FL, Dutilh BE, Nieuwdorp M, Herrema H (2022) Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nat Commun 13(1) https://doi.org/10.1038/s41467-022-31390-5
De la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS (2018) Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 11(1):51
De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G (2016) Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 24(1):151–157. https://doi.org/10.1016/j.cmet.2016.06.013
Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, Zhang Z, Bakal JA, Walter J (2020) Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27(3):389-404.e6
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM (2019) Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25(7):1096–1103
Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A (2016) A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167(5):1339-1353.e21
Díez-Ricote L, Ruiz-Valderrey P, Micó V, Blanco R, Tomé-Carneiro J, Dávalos A, Ordovás JM, Daimiel L (2022) TMAO upregulates members of the miR-17/92 cluster and impacts targets associated with atherosclerosis. Int J Mol Sci 23(20):12107
Ding L, Xu Z-J, Shi H-H, Xue C-H, Huang Q-R, Yanagita T, Wang Y-M, Zhang T-T (2021) Sterol sulfate alleviates atherosclerosis via mediating hepatic cholesterol metabolism in ApoE−/− mice. Food Funct 12(11):4887–4896
Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA (2017) A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551(7682):648–652
Dong Y, Xu M, Chen L, Bhochhibhoya A (2019) Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and meta-analysis of recent clinical trials. Ann Nutr Metab 74(3):224–241
Du L, Li Q, Yi H, Kuang T, Tang Y, Fan G (2022) Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed Pharmacother 149 https://doi.org/10.1016/j.biopha.2022.112839
Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Fito M, Gea A, Hernan MA, Martinez-Gonzalez MA, Investigators PS (2018) Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. New Engl J Med 378(25) https://doi.org/10.1056/NEJMoa1800389
Fang C, Zuo K, Jiao K, Zhu X, Fu Y, Zhong J, Xu L, Yang X (2022) PAGln, an atrial fibrillation-linked gut microbial metabolite, acts as a promoter of atrial myocyte injury. Biomolecules 12(8):1120
Feng W, Liu J, Cheng H, Zhang D, Tan Y, Peng C (2022) Dietary compounds in modulation of gut microbiota-derived metabolites. Front Nutr 9:939571. https://doi.org/10.3389/fnut.2022.939571
Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F (2019) Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. Plos One 14(6) https://doi.org/10.1371/journal.pone.0218384
Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, Chrysochoou CA, Nihoyannopoulos PI, Tousoulis DM (2020) Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 11(5):1150–1160
Frampton J, Murphy KG, Frost G, Chambers ES (2020) Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab 2(9):840–848. https://doi.org/10.1038/s42255-020-0188-7
Gan J, Dou Y, Li Y, Wang Z, Wang L, Liu S, Li Q, Yu H, Liu C, Han C (2018) Producing anti-inflammatory macrophages by nanoparticle-triggered clustering of mannose receptors. Biomaterials 178:95–108
Gao R, Meng X, Xue Y, Mao M, Liu Y, Tian X, Sui B, Li X, Zhang P (2022) Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front Pharmacol 13 https://doi.org/10.3389/fphar.2022.1027212
Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC (2018) Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol 9:890
Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, ** X, Zhou X, Fan H (2020) The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose–response meta-analysis. Adv Nutr 11(1):66–76
Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, Li J (2018) Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother 97:941–947
Griffin JL, Wang X, Stanley E (2015) Does our gut microbiome predict cardiovascular risk? A review of the evidence from metabolomics. Circ-Cardiovasc Gene 8(1):187–191
Guo M, Fan X, Tuerhongjiang G, Wang C, Wu H, Lou B, Wu Y, Yuan Z, She J (2021) Targeted metabolomic analysis of plasma fatty acids in acute myocardial infarction in young adults. Nutr Metab Cardiovas 31(11):3131–3141
Guo ZN, Pan JJ, Zhu HY, Chen ZY (2022) Metabolites of gut microbiota and possible implication in development of diabetes mellitus. J Agric Food Chem 70(20):5945–5960. https://doi.org/10.1021/acs.jafc.1c07851
Gutierrez-Mariscal FM, de la Cruz-Ares S, Torres-Peña JD, Alcalá-Diaz JF, Yubero-Serrano EM, López-Miranda J (2021) Coenzyme q10 and cardiovascular diseases. Antioxidants 10(6):906
Hadi A, Alizadeh K, Hajianfar H, Mohammadi H, Miraghajani M (2020) Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit Rev Food Sci 60(4):584–596
Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, Heinze P, Kaisler J, Nageswaran V, Aigner A, Ceglarek U, Cineus R, Hegazy AN, van der Vorst EPC, Doring Y, Strauch CM, Nemet I, Tremaroli V, Dwibedi C, Krankel N, Leistner DM, Heimesaat MM, Bereswill S, Rauch G, Seeland U, Soehnlein O, Mueller DN, Gold R, Baeckhed F, Hazen SL, Haghikia A, Landmesser U (2022) Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J 43(6):518–533. https://doi.org/10.1093/eurheartj/ehab644
He GD, Liu XC, Lu AS, Feng YQ (2022) Association of choline intake with blood pressure and effects of its microbiota-dependent metabolite trimethylamine-N-oxide on hypertension. Cardiovasc Ther 2022 https://doi.org/10.1155/2022/9512401
Hendijani F, Akbari V (2018) Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr (edinburgh, Scotland) 37(2):532–541
Hodge RJ, Nunez DJ (2016) Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes Metab 18(5):439–443. https://doi.org/10.1111/dom.12636
Hoving LR, Katiraei S, Pronk A, Hei**k M, Vonk KKD, Amghar-el Bouazzaoui F, Vermeulen R, Drinkwaard L, Giera M, van Harmelen V, van Dijk KW (2018) The prebiotic inulin modulates gut microbiota but does not ameliorate atherosclerosis in hypercholesterolemic APOE*3-Leiden. CETP mice. Sci Rep-UK 8https://doi.org/10.1038/s41598-018-34970-y
Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, Silver PA, Gerber GK (2019) Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 25(6):803–814
Hsu C-N, Hou C-Y, Lee C-T, Chang-Chien G-P, Lin S, Tain Y-L (2021) Maternal 3, 3-dimethyl-1-butanol therapy protects adult male rat offspring against hypertension programmed by perinatal TCDD exposure. Nutrients 13(9):3041
Hu S, Chen Y, Zhao S, Sun K, Luo L, Zeng L (2021) Ripened Pu-Erh tea improved the enterohepatic circulation in a circadian rhythm disorder mice model. J Agric Food Chem 69(45):13533–13545. https://doi.org/10.1021/acs.jafc.1c05338
Hu T, Wu Q, Yao Q, Jiang K, Yu J, Tang Q (2022b) Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev 81:101706–101706. https://doi.org/10.1016/j.arr.2022.101706
Hu T, Wu Q, Yao Q, Jiang K, Yu J, Tang Q (2022a) Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev 101706
Huang YH, Lin FJ, Tang RD, Bao CL, Zhou QX, Ye KW, Shen Y, Liu CL, Hong C, Yang K, Tang HY, Wang J, Lu WJ, Wang T (2022b) Gut microbial metabolite trimethylamine N-oxide aggravates pulmonary hypertension. Am J Resp Cell Mol 66(4):452–460. https://doi.org/10.1165/rcmb.2021-0414OC
Huang Y, Zhang H, Fan X, Wang J, Yin Y, Zhang Y, Shi K, Yu F (2022a) The role of gut microbiota and trimethylamine N-oxide in cardiovascular diseases. J Cardiovasc Transl 1–9
Huart J, Cirillo A, Taminiau B, Descy J, Saint-Remy A, Daube G, Krzesinski J-M, Melin P, De Tullio P, Jouret F (2021) Human stool metabolome differs upon 24 h blood pressure levels and blood pressure dip** status: a prospective longitudinal study. Metabolites 11(5):282
Hug H, Mohajeri MH, La Fata G (2018) Toll-like receptors: regulators of the immune response in the human gut. Nutrients 10(2):203
Iglesias-Carres L, Hughes MD, Steele CN, Ponder MA, Davy KP, Neilson AP (2021) Use of dietary phytochemicals for inhibition of trimethylamine N-oxide formation. J Nutr Biochem 91:108600
Innes JK, Calder PC (2020) Marine Omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci 21(4) https://doi.org/10.3390/ijms21041362
Ishimwe JA, Dola T, Ertuglu LA, Kirabo A (2022) Bile acids and salt-sensitive hypertension: a role of the gut-liver axis. Am J Physiol-Heart C 322(4):H636–H646
Israr MZ, Bernieh D, Salzano A, Cassambai S, Yazaki Y, Heaney LM, Jones DJL, Ng LL, Suzuki T (2021) Association of gut-related metabolites with outcome in acute heart failure. Am Heart J 234:71–80. https://doi.org/10.1016/j.ahj.2021.01.006
Jackson MA, Verdi S, Maxan M-E, Shin CM, Zierer J, Bowyer RC, Martin T, Williams FM, Menni C, Bell JT (2018) Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 9(1):1–8
Jadhav K, Xu Y, Xu Y, Li Y, Xu J, Zhu Y, Adorini L, Lee YK, Kasumov T, Yin L (2018) Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol Metab 9:131–140
Jama HA, Beale A, Shihata WA, Marques FZ (2019a) The effect of diet on hypertensive pathology: is there a link via gut microbiota-driven immunometabolism? Cardiovasc Res 115(9):1435–1447
Jama HA, Kaye DM, Marques FZ (2019b) The gut microbiota and blood pressure in experimental models. Curr Opin Nephrol Hy 28(2):97–104. https://doi.org/10.1097/mnh.0000000000000476
Javed I, Cui X, Wang X, Mortimer M, Andrikopoulos N, Li Y, Davis TP, Zhao Y, Ke PC, Chen C (2020) Implications of the human gut–brain and gut–cancer axes for future nanomedicine. ACS Nano 14(11):14391–14416
Jiang X, Sun B, Zhou Z (2022) Preclinical studies of natural products targeting the gut microbiota: beneficial effects on diabetes. J Agric Food Chem 70(28):8569–8581. https://doi.org/10.1021/acs.jafc.2c02960
** L, Shi X, Yang J, Zhao Y, Xue L, Xu L, Cai J (2021) Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 12(5):346–359
Johnson SA, Weir TL (2022) Fresh take on the relationship between diet, gut microbiota, and atherosclerosis: a food-based approach with Brussels chicory. J Nutr 152(10):2181–2183
Jonsson AL, Bäckhed F (2017) Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 14(2):79–87
Juraschek SP, Kovell LC, Appel LJ, Miller ER III, Sacks FM, Christenson RH, Rebuck H, Chang AR, Mukamal KJ (2020) Associations between dietary patterns and subclinical cardiac injury: an observational analysis from the DASH trial. Ann Intern Med 172(12):786–794
Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, Horlock D, Vijay A, Giam B, Vinh A, Johnson C, Fiedler A, Donner D, Snelson M, Coughlan MT, Phillips S, Du XJ, El-Osta A, Drummond G, Lambert GW, Spector TD, Valdes AM, Mackay CR, Marques FZ (2020) Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 141(17):1393–1403. https://doi.org/10.1161/circulationaha.119.043081
Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S (2020) Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome 8(1):1–17
Kirschner SK, Deutz NEP, Rijnaarts I, Smit TJ, Larsen DJ, Engelen MPKJ (2022) Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. Jpen-Parenter Enter 46(3):660–670. https://doi.org/10.1002/jpen.2193
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19(5):576–585
Koh A, Backhed F (2020) From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell 78(4):584–596. https://doi.org/10.1016/j.molcel.2020.03.005
Kummen M, Mayerhofer CC, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, Ueland T, Yndestad A, Hov JR, Trøseid M (2018) Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol 71(10):1184–1186
Lázaro I, Lupón J, Cediel G, Codina P, Fitó M, Domingo M, Santiago-Vacas E, Zamora E, Sala-Vila A, Bayés-Genís A (2022) Relationship of circulating vegetable omega-3 to prognosis in patients with heart failure. J Am Coll Cardiol 80(18):1751–1758
Lee P, Yacyshyn BR, Yacyshyn MB (2019) Gut microbiota and obesity: an opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes Metab 21(3):479–490
Leustean AM, Ciocoiu M, Sava A, Costea CF, Floria M, Tarniceriu CC, Tanase DM (2018) Implications of the intestinal microbiota in diagnosing the progression of diabetes and the presence of cardiovascular complications. J Diabetes Res 2018:5205126
Lewis CV, Taylor WR (2020) Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am J Physiol-Heart C 319(6):H1227–H1233. https://doi.org/10.1152/ajpheart.00612.2020
Lewis-Mikhael AM, Davoodvandi A, Jafarnejad S (2020) Effect of Lactobacillusplantarum containing probiotics on blood pressure: a systematic review and meta-analysis. Pharmacol Res 153:104663
Li J, Dawson PA (2019) Animal models to study bile acid metabolism. Bba-Mol Basis Dis 1865(5):895–911. https://doi.org/10.1016/j.bbadis.2018.05.011
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M (2019) Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 99(3):346–357
Li C, Yang J, Wang Y, Qi Y, Yang W, Li Y (2020a) Farnesoid X receptor agonists as therapeutic target for cardiometabolic diseases. Front Pharmacol 11:1247
Li DD, Lu Y, Yuan S, Cai XX, He Y, Chen J, Wu Q, He D, Fang AP, Bo YC, Song PG, Bogaert D, Tsilidis K, Larsson SC, Yu HL, Zhu HL, Theodoratou E, Zhu YM, Li X (2022a) Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis. Am J Clin Nutr 116(1):230–243. https://doi.org/10.1093/ajcn/nqac074
Li DF, Yang MF, Xu J, Xu HM, Zhu MZ, Liang YJ, Zhang Y, Tian CM, Nie YQ, Shi RY, Wang LS, Yao J (2022b) Extracellular vesicles: the next generation theranostic nanomedicine for inflammatory bowel disease. Int J Nanomed 17:3893–3911. https://doi.org/10.2147/ijn.S370784
Li Q, Zhang X, Du YS, Liu XP, Chen GY, **ang PY, Wu H, Liu CQ, Wang DL (2022c) Brussels chicory stabilizes unstable atherosclerotic plaques and reshapes the gut microbiota in Apoe(-/-) mice. J Nutr 152(10):2209–2217. https://doi.org/10.1093/jn/nxac103
Li W, Chen C, Chen M, Zhang X, Ji Q, Wang Y, Zheng Q, Tan S, Gao X, Lu Y (2020b) Salted and unsalted Zhacai (Brassica juncea var. tumida) alleviated high-fat diet-induced dyslipidemia by regulating gut microbiota: a multiomics study. Mol Nutr Food Res 64(24) https://doi.org/10.1002/mnfr.202000798
Li SY, Chen S, Lu XT, Fang AP, Chen YM, Huang RZ, Lin XL, Huang ZH, Ma JF, Huang BX, Zhu HL (2022d) Serum trimethylamine-N-oxide is associated with incident type 2 diabetes in middle-aged and older adults: a prospective cohort study. 74 20(1) https://doi.org/10.1186/s12967-022-03581-7
Liang X, Zhang Z, Lv Y, Tong L, Liu T, Yi H, Zhou X, Yu Z, Tian X, Cui Q (2020) Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis. Nutrition 79:110941
Liang T, Wu L, ** Y, Li Y, **e X, Fan C, Yang L, Yang S, Chen X, Zhang J (2020) Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: an update of meta-analysis. Crit Rev Food Sci 61(10):1670–1688
Liu X, Morris MC, Dhana K, Ventrelle J, Johnson K, Bishop L, Hollings CS, Boulin A, Laranjo N, Stubbs BJ (2021a) Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp Clin Trials 102:106270
Liu Y, Lou X (2020) Type 2 diabetes mellitus-related environmental factors and the gut microbiota: emerging evidence and challenges. Clinics (Sao Paulo) 75:e1277
Liu Y, Jiang Q, Liu Z, Shen S, Ai J, Zhu Y, Zhou L (2021b) Alteration of gut microbiota relates to metabolic disorders in primary aldosteronism patients. Front Endocrinol 12 https://doi.org/10.3389/fendo.2021.667951
Loscalzo J (2013) Gut microbiota, the genome, and diet in atherogenesis. Mass Medical Soc 368:1647–1649
Lu Y, Zhang Y, Zhao X, Shang C, **ang M, Li L, Cui X (2022) Microbiota-derived short-chain fatty acids: implications for cardiovascular and metabolic disease. Front Cardiovasc Med 9 https://doi.org/10.3389/fcvm.2022.900381
Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, Lieb W, Franke A, Ott SJ, Frey N (2017) Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Failure 4(3):282–290
Luo Q, Hu Y, Chen X, Luo Y, Chen J, Wang H (2022) Effects of gut microbiota and metabolites on heart failure and its risk factors: a two-sample Mendelian randomization study. Front Nutr 9:899746. https://doi.org/10.3389/fnut.2022.899746
Ma J, Li H (2018) The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol 9:1082
Mahmoudi M (2018) Debugging nano-bio interfaces: systematic strategies to accelerate clinical translation of nanotechnologies. Trends Biotechnol 36(8):755–769. https://doi.org/10.1016/j.tibtech.2018.02.014
Manolis AA, Manolis TA, Melita H, Manolis AS (2022) Gut microbiota and cardiovascular disease: symbiosis versus dysbiosis. Curr Med Chem 29(23):4050–4077
Margina D, Ungurianu A, Purdel C, Nitulescu GM, Tsoukalas D, Sarandi E, Thanasoula M, Burykina TI, Tekos F, Buha A, Nikitovic D, Kouretas D, Tsatsakis AM (2020) Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem Toxicol 143 https://doi.org/10.1016/j.fct.2020.111558
Martin AM, Sun EW, Rogers GB, Keating DJ (2019) The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front Physiol 10:428
Martínez-González MA, Gea A, Ruiz-Canela M (2019) The Mediterranean diet and cardiovascular health: a critical review. Circ Res 124(5):779–798
Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, Aukrust P, Gullestad L, Hov JR, Troseid M (2017) Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail 23(9):666–671. https://doi.org/10.1016/j.cardfail.2017.06.007
Mei Z, Chen G-C, Wang Z, Usyk M, Yu B, Baeza YV, Humphrey G, Benitez RS, Li J, Williams-Nguyen JS (2021) Dietary factors, gut microbiota, and serum trimethylamine-N-oxide associated with cardiovascular disease in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr 113(6):1503–1514
Millet YA, Meisner J, Tan J, Jose A, Humphries E, Miller KJ, Bayne C, McComb M, Giuggio M, Konopnicki CM (2022) Modulation of the gut microbiome by novel synthetic glycans for the production of propionate and the reduction of cardiometabolic risk factors. bioRxiv 2022–04
Min L, Qinghua H, **glu Y (2018) Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin Exp Hypertens 41(4):1–11
Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S (2019) Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol 185
Moran-Ramos S, López-Contreras BE, Canizales-Quinteros S (2017) Gut microbiota in obesity and metabolic abnormalities: a matter of composition or functionality? Arch Med Res 48(8):735–753
Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM (1996) Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 77(9):723–727. https://doi.org/10.1016/s0002-9149(97)89206-5
Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM, Cajka T, Mohan ML, Li L, Wu Y (2020) A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 180(5):862–877
O’Morain VL, Ramji DP (2020) The potential of probiotics in the prevention and treatment of atherosclerosis. Mol Nutr Food Res 64(4):1900797
Opoku-Acheampong I, McLaud T, Anderson OS (2022) Fecal microbiota transplantation to prevent and treat chronic disease: implications for dietetics practice. J Acad Nutr Diet 122(1):33–37
Osuna-Prieto FJ, Rubio-Lopez J, Di X, Yang W, Kohler I, Rensen PC, Ruiz JR, Martinez-Tellez B (2022) Plasma levels of bile acids are related to cardiometabolic risk factors in young adults. J Clin Endocrinology Metabolism 107(3):715–723
Pakhomov N, Baugh JA (2021) The role of diet-derived short-chain fatty acids in regulating cardiac pressure overload. Am J Physiol-Heart C 320(2):H475–H486. https://doi.org/10.1152/ajpheart.00573.2020
Palmu J, Salosensaari A, Havulinna AS, Cheng S, Inouye M, Jain M, Salido RA, Sanders K, Brennan C, Humphrey GC (2020) Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J Am Heart Assoc 9(15):e016641
Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, Menichelli D, Pignatelli P, Violi F (2017) Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: Effect of adherence to mediterranean diet. J Am Heart Assoc 6(6):e005784
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Dore J, Mattila I, Plichta DR, Poho P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jorgensen T, Holm JB, Trost K, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O, Meta HITC (2016) Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535(7612):376. https://doi.org/10.1038/nature18646
Qato DM, Ozenberger K, Olfson M (2018) Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. JAMA 319(22):2289–2298
Qi D, Nie X-L, Zhang J-J (2020) The effect of probiotics supplementation on blood pressure: a systemic review and meta-analysis. Lipids Health Dis 19(1):1–11
Qi S, Luo X, Liu S, Ling B, ** H (2022) The critical effect of bile acids in atherosclerosis. J Cardiovasc Pharm 80(4):562–573. https://doi.org/10.1097/fjc.0000000000001320
Quek J, Lim G, Lim WH, Ng CH, So WZ, Toh J, Pan XH, Chin YH, Muthiah MD, Chan SP (2021) The association of plant-based diet with cardiovascular disease and mortality: a meta-analysis and systematic review of prospect cohort studies. Front Cardiovasc Med 8
Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJS, Anker SD (2000) Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 102(25):3060–3067
Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A, Das A, Hartley L, Stranges S (2019) Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Db Syst Rev (3)
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341(6150):1241214
Robles-Vera I, Toral M, de la Visitacion N, Sanchez M, Gomez-Guzman M, Munoz R, Algieri F, Vezza T, Jimenez R, Galvez J, Romero M, Miguel Redondo J, Duarte J (2020) Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Brit J Pharmacol 177(9):2006–2023. https://doi.org/10.1111/bph.14965
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 76(25):2982–3021
Salas-Salvadó J, Becerra-Tomás N, García-Gavilán JF, Bullo M, Barrubes L (2018) Mediterranean diet and cardiovascular disease prevention: what do we know? Prog Cardiovasc Dis 61(1):62–67
Sata Y, Marques FZ, Kaye DM (2020) The emerging role of gut dysbiosis in cardio-metabolic risk factors for heart failure. Curr Hypertens Rep 22(5):1–8
Schellekens H, Torres-Fuentes C, van de Wouw M, Long-Smith CM, Mitchell A, Strain C, Berding K, Bastiaanssen TF, Rea K, Golubeva AV (2021) Bifidobacterium longum counters the effects of obesity: partial successful translation from rodent to human. EBioMedicine 63:103176
Shah RV, Steffen LM, Nayor M, Reis JP, Jacobs DR, Allen NB, Lloyd-Jones D, Meyer K, Cole J, Piaggi P (2022) Dietary metabolic signatures and cardiometabolic risk. Eur Heart J
Shkoporov AN, Hill C (2019) Bacteriophages of the human gut: the “known unknown” of the microbiome. Cell Host Microbe 25(2):195–209
Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MC, Kemper EM (2018) Effect of vegan fecal microbiota transplantation on carnitine-and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc 7(7):e008342
Sun LJ, Ma LJ, Ma YB, Zhang FM, Zhao CH, Nie YZ (2018) Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 9(5):397–403. https://doi.org/10.1007/s13238-018-0546-3
Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR Jr, Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA (2019) Gut microbiota composition and blood pressure: the CARDIA study. Hypertension 73(5):998–1006
Suzuki T, Yazaki Y, Voors AA, Jones DJ, Chan DC, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL (2019) Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: results from BIOSTAT-CHF. Eur J Heart Fail 21(7):877–886
Szczepańska E, Białek-Dratwa A, Janota B, Kowalski O (2022) Dietary therapy in prevention of cardiovascular disease (CVD)—tradition or modernity? A review of the latest approaches to nutrition in CVD. Nutrients 14(13):2649
Tang W, Li DY, Hazen SL (2019a) Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol 16(3):137–154
Tang WW, Bäckhed F, Landmesser U, Hazen SL (2019b) Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol 73(16):2089–2105
Tang WHW, Li XS, Wu YP, Wang ZN, Khaw KT, Wareham NJ, Nieuwdorp M, Boekholdt SM, Hazen SL (2021) Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the EPIC-Norfolk prospective population study. Am Heart J 236:80–86. https://doi.org/10.1016/j.ahj.2021.01.020
Tang Y, Zou Y, Cui J, Ma X, Zhang L, Yu S, Qiu L (2022) Analysis of two intestinal bacterial metabolites (trimethylamine N-oxide and phenylacetylglutamine) in human serum samples of patients with T2DM and AMI using a liquid chromatography tandem mass spectrometry method. Clin Chim Acta 536:162–168
Testai L, Martelli A, Flori L, Cicero AF, Colletti A (2021) Coenzyme Q10: clinical applications beyond cardiovascular diseases. Nutrients 13(5):1697
Thingholm LB, Rühlemann MC, Koch M, Fuqua B, Laucke G, Boehm R, Bang C, Franzosa EA, Hübenthal M, Rahnavard A (2019) Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe 26(2):252-264.e10
Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF (2017) The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol 2(10):747–756
Trautwein EA, McKay S (2020) The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients 12(9):2671
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y (2022) Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 145(8):e153–e639
Turnbaugh PJ (2020) Diet should be a tool for researchers, not a treatment. Nature 577(7792):S23–S23
Verhaar BJ, Collard D, Prodan A, Levels JH, Zwinderman AH, Bäckhed F, Vogt L, Peters MJ, Muller M, Nieuwdorp M (2020) Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J 41(44):4259–4267
Walter J, Armet AM, Finlay BB, Shanahan F (2020) Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell 180(2):221–232
Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, Lucia S, Adorini L, D’Agati VD, Levi J (2016) G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol 27(5):1362–1378
Wang H, He Q, Wang G, Xu X, Hao H (2018a) FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat 28(11):765–782
Wang Y, Li L, Zhao W, Dou Y, An H, Tao H, Xu X, Jia Y, Lu S, Zhang J (2018b) Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 12(9):8943–8960
Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WW (2019) Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 40(7):583–594
Wang DD, Nguyen LH, Li Y, Yan Y, Ma W, Rinott E, Ivey KL, Shai I, Willett WC, Hu FB (2021a) The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med 27(2):333–343
Wang F, Zhao C, Yang M, Zhang L, Wei R, Meng K, Bao Y, Zhang L, Zheng J (2021b) Four citrus flavanones exert atherosclerosis alleviation effects in ApoE–/–mice via different metabolic and signaling pathways. J Agric Food Chem 69(17):5226–5237
Wang Z, Cheng C, Yang X, Zhang C (2021c) L-phenylalanine attenuates high salt-induced hypertension in Dahl SS rats through activation of GCH1-BH4. PLoS One 16(4):e0250126
Wang ZC, Wu F, Zhou QB, Qiu YM, Zhang JN, Tu Q, Zhou Z, Shao YJ, Xu SY, Wang Y, Tao J (2022) Berberine improves vascular dysfunction by inhibiting trimethylamine-N-oxide via regulating the gut microbiota in angiotensin ii-induced hypertensive mice. Front Microbiol 13 https://doi.org/10.3389/fmicb.2022.814855
Wei H, Zhao M, Huang M, Li C, Gao J, Yu T, Zhang Q, Shen X, Ji L, Ni L, Zhao C, Wang Z, Dong E, Zheng L, Wang DW (2022) FMO3-TMAO axis modulates the clinical outcome in chronic heart-failure patients with reduced ejection fraction: evidence from an Asian population. Front Med-PRC 16(2):295–305. https://doi.org/10.1007/s11684-021-0857-2
White PJ, Newgard CB (2019) Branched-chain amino acids in disease. Science 363(6427):582–583
Wijdeveld M, Nieuwdorp M, Ijzerman R (2020) The interaction between microbiome and host central nervous system: the gut-brain axis as a potential new therapeutic target in the treatment of obesity and cardiometabolic disease. Expert Opin Ther Tar 24(7):639–653
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052):105–108
Wu T-R, Lin C-S, Chang C-J, Lin T-L, Martel J, Ko Y-F, Ojcius DM, Lu C-C, Young JD, Lai H-C (2019) Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 68(2):248–262
Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, Gummesson A, Perkins R, Bergström G, Bäckhed F (2020) The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab 32(3):379-390.e3
Wu Q, Sun L, Hu X, Wang X, Xu F, Chen B, Liang X, **a J, Wang P, Aibara D (2021) Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J Clin Invest 131(9)
Wu L, Li M-Q, **e Y-T, Zhang Q, Lu X-J, Liu T, Lin W-Y, Xu J-T, Wu Q-P, He X-X (2022a) Washed microbiota transplantation improves patients with high blood glucose in South China. Front Endocrinol 13 https://doi.org/10.3389/fendo.2022.985636
Wu L, Lu X-J, Lin D-J, Chen W-J, Xue X-Y, Liu T, Xu J-T, **e Y-T, Li M-Q, Lin W-Y (2022b) Washed microbiota transplantation improves patients with metabolic syndrome in South China. Front Cell Infect MI 1713
Xu J, Yang Y (2020) Implications of gut microbiome on coronary artery disease. Cardiovasc Diagn The 10(4):869
Xu J, Yang Y (2021) Gut microbiome and its meta-omics perspectives: profound implications for cardiovascular diseases. Gut Microbes 13(1):1936379
Yan S, Tian Z, Li M, Li B, Cui W (2019) Effects of probiotic supplementation on the regulation of blood lipid levels in overweight or obese subjects: a meta-analysis. Food Funct 10(3):1747–1759
Yan X, ** J, Su X, Yin X, Gao J, Wang X, Zhang S, Bu P, Wang M, Zhang Y, Wang Z, Zhang Q (2020) Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res 126(7):839–853
Yang JJ, Shu X-O, Herrington DM, Moore SC, Meyer KA, Ose J, Menni C, Palmer ND, Eliassen H, Harada S (2021b) Circulating trimethylamine N-oxide in association with diet and cardiometabolic biomarkers: an international pooled analysis. Am J Clin Nutr 113(5):1145–1156
Yang WQ, Ren DY, Zhao Y, Liu L, Yang XB (2021d) Fuzhuan brick tea polysaccharide improved ulcerative colitis in association with gut microbiota-derived tryptophan metabolism. J Agric Food Chem 69(30):8448–8459. https://doi.org/10.1021/acs.jafc.1c02774
Yang Z-H, Liu F, Zhu X-R, Suo F-Y, Jia Z-j, Yao S-K (2021e) Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J Gastroentero 27(24):3609
Yang Y, Zeng Q, Gao J, Yang B, Zhou J, Li K, Li L, Wang A, Li X, Liu Z, Luo Q, Zhao Z, Liu B, Xue J, Jiang X, Konerman MC, Zheng L, **ong C (2022) High-circulating gut microbiota-dependent metabolite trimethylamine N-oxide is associated with poor prognosis in pulmonary arterial hypertension. Eur Heart J Open 2(5):oeac021. https://doi.org/10.1093/ehjopen/oeac021
Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, Meng L, **n Y, Jiang X (2021a) Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 117 https://doi.org/10.1016/j.metabol.2021.154712
Yang S, Li D, Yu Z, Li Y, Wu M (2021c) Multi-pharmacology of berberine in atherosclerosis and metabolic diseases: potential contribution of gut microbiota. Front Pharmacol 12 https://doi.org/10.3389/fphar.2021.709629
Yao Y, Yan L, Chen H, Wu N, Wang W, Wang D (2020) Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 77 https://doi.org/10.1016/j.phymed.2020.153268
Yoo W, Zieba JK, Foegeding NJ, Torres TP, Shelton CD, Shealy NG, Byndloss AJ, Cevallos SA, Gertz E, Tiffany CR (2021) High-fat diet–induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 373(6556):813–818
Yoo JY, Sniffen S, McGill Percy KC, Pallaval VB, Chidipi B (2022) Gut dysbiosis and immune system in atherosclerotic cardiovascular disease (ACVD). Microorganisms 10(1) https://doi.org/10.3390/microorganisms10010108
Yousefi B, Kokhaei P, Mehranfar F, Bahar A, Abdolshahi A, Emadi A, Eslami M (2022) The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Eurosci Biobehav R 132:998–1009. https://doi.org/10.1016/j.neubiorev.2021.10.046
Yuan Q, **n L, Han S, Su Y, Wu R, Liu X, Wuri J, Li R, Yan T (2021) Lactulose improves neurological outcomes by repressing harmful bacteria and regulating inflammatory reactions in mice after stroke. Front Cell Infect Mi 11 https://doi.org/10.3389/fcimb.2021.644448
Yuan L, Li Y, Chen M, Xue L, Wang J, Ding Y, Zhang J, Wu S, Ye Q, Zhang S, Yang R, Zhao H, Wu L, Liang T, **e X, Wu Q (2022) Antihypertensive activity of milk fermented by Lactiplantibacillus plantarum SR37-3 and SR61-2 in L-NAME-induced hypertensive rats. Foods 11(15) 10.3390/foods11152332
Yukino-Iwashita M, Nagatomo Y, Kawai A, Taruoka A, Yumita Y, Kagami K, Yasuda R, Toya T, Ikegami Y, Masaki N, Ido Y, Adachi T (2022) Short-chain fatty acids in gut-heart axis: their role in the pathology of heart failure. J Pers Med 12(11) https://doi.org/10.3390/jpm12111805
Zeng S-L, Li S-Z, **ao P-T, Cai Y-Y, Chu C, Chen B-Z, Li P, Li J, Liu E-H (2020) Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci Adv 6(1):eaax6208
Zhang F, Yuan W, Wei Y, Zhang D, Duan Y, Li B, Wang X, ** L, Zhou Y, Wu X (2019a) The alterations of bile acids in rats with high-fat diet/streptozotocin-induced type 2 diabetes and their negative effects on glucose metabolism. Life Sci 229:80–92. https://doi.org/10.1016/j.lfs.2019.05.031
Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, Walter J, Madsen KL (2019b) Impact of fecal microbiota transplantation on obesity and metabolic syndrome—a systematic review. Nutrients 11(10):2291
Zhang S, Liang Y, Li L, Chen Y, Wu P, Wei D (2022) Succinate: a novel mediator to promote atherosclerotic lesion progression. DNA Cell Biol 41(3):285–291. https://doi.org/10.1089/dna.2021.0345
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359(6380):1151–1156
Zhao YH, Li MJ, Wang YN, Geng RX, Fang JJ, Liu Q, Kang SG, Zeng WC, Huang KL, Tong T (2022) Understanding the mechanism underlying the anti-diabetic effect of dietary component: a focus on gut microbiota. Crit Rev Food Sci. https://doi.org/10.1080/10408398.2022.2045895
Zheng L, Ji Y-Y, Wen X-L, Duan S-L (2022) Fecal microbiota transplantation in the metabolic diseases: current status and perspectives. World J Gastroentero 28(23):2546
Zhong HJ, Zeng HL, Cai YL, Zhuang YP, Liou YL, Wu Q (2021) He XX (2021) Washed microbiota transplantation lowers blood pressure in patients with hypertension. Front Cell Infect Mi 11(2021):679624
Zhou W, Sailani MR, Contrepois K, Zhou Y, Ahadi S, Leopold SR, Zhang MJ, Rao V, Avina M, Mishra T, Johnson J, Lee-McMullen B, Chen S, Metwally AA, Tran TDB, Nguyen H, Zhou X, Albright B, Hong B-Y, Petersen L, Bautista E, Hanson B, Chen L, Spakowicz D, Bahmani A, Salins D, Leopold B, Ashland M, Dagan-Rosenfeld O, Rego S, Limcaoco P, Colbert E, Allister C, Perelman D, Craig C, Wei E, Chaib H, Hornburg D, Dunn J, Liang L, Rose SMS-F, Kukurba K, Piening B, Rost H, Tse D, McLaughlin T, Sodergren E, Weinstock GM, Snyder M (2019) Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 569(7758):663–671. https://doi.org/10.1038/s41586-019-1236-x
Zhou X, ** MC, Liu L, Yu ZL, Lu X, Zhang H (2020) Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. Esc Heart Fail 7(1):189–194. https://doi.org/10.1002/ehf2.12552
Zhou M, Li D, **e K, Xu L, Kong B, Wang X, Tang Y, Liu Y, Huang H (2021) The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct 12(24):12580–12593
Zhu Y, Li Q, Jiang H (2020) Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide. Apmis 128(5):353–366
Zhu Z, Huang R, Liu W, Wang J, Wu S, Chen M, Huang A, **e Y, Chen M, Jiao C (2022) Whole agrocybe cylindracea prevented obesity linking with modification of gut microbiota and associated fecal metabolites in high-fat diet-fed mice. Mol Nutr Food Res 66(7):2100897
Zong X, Fan Q, Yang Q, Pan R, Zhuang L, Tao R (2022) Phenylacetylglutamine as a risk factor and prognostic indicator of heart failure. ESC Heart Failure 9(4):2645–2653
Funding
This research was jointly funded by Guangdong Province National key research and development project (2021YFA0910200), Guangdong Province key research and development project (2022B1111070006), and Innovation Development Project of Guangdong Academy of Sciences (2020GDASYL20200102003).
Author information
Authors and Affiliations
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, L., Li, Y., Chen, M. et al. Therapeutic applications of gut microbes in cardiometabolic diseases: current state and perspectives. Appl Microbiol Biotechnol 108, 156 (2024). https://doi.org/10.1007/s00253-024-13007-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13007-7