Abstract
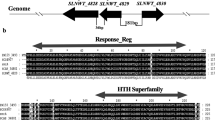
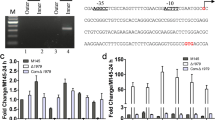
Two-component system AfsQ1-Q2 of Streptomyces coelicolor was identified previously for its ability to stimulate actinorhodin (ACT) and undecylprodigiosin (RED) production in Streptomyces lividans. However, disruption of either afsQ1 or afsQ2 in S. coelicolor led to no detectable changes in secondary metabolite formation or morphogenesis. In this study, we reported that, when cultivated on defined minimal medium (MM) with glutamate as the sole nitrogen source, the afsQ mutant exhibited significantly decreased ACT, RED, and calcium-dependent antibiotic (CDA) production and rapid growth of aerial mycelium. In addition, we also found that deletion of sigQ, which is located upstream of afsQ1-Q2 and encodes a putative sigma factor, led to the precocious hyperproduction of these antibiotics and delayed formation of sporulating aerial mycelium in the same glutamate-based defined MM. Reverse-transcription polymerase chain reaction and egfp fusion analyses showed that the expression of sigQ was under control by afsQ. In addition, deletion of both afsQ-sigQ resulted in the phenotype identical to that of afsQ mutant. The results suggested that afsQ1-Q2 and sigQ worked together in the regulation of both antibiotic biosynthesis and morphological development, and sigQ might be responsible for antagonizing the function of AfsQ1-Q2 in S. coelicolor, however, in a medium-dependent manner. Moreover, the study showed that the medium-dependent regulation of antibiotic biosynthesis by AfsQ1-Q2-SigQ was through pathway-specific activator genes actII-ORF4, redD, and cdaR. The study provides new insights on regulation of antibiotic biosynthesis and morphological development in S. coelicolor.





Similar content being viewed by others
References
Aceti DJ, Champness WC (1998) Transcriptional regulation of Streptomyces coelicolor pathway-specific antibiotic regulators by the absA and absB loci. J Bacteriol 180:3100–3106
Adamidis T, Riggle P, Champness W (1990) Mutations in a new Streptomyces coelicolor locus which globally block antibiotic biosynthesis but not sporulation. J Bacteriol 172:2962–2969
Anderson T, Brian P, Riggle P, Kong RQ, Champness W (1999) Genetic suppression analysis of non-antibiotic-producing mutants of the Streptomyces coelicolor absA locus. Microbiology 145:2343–2353
Anderson TB, Brian P, Champness WC (2001) Genetic and transcriptional analysis of absA, an antibiotic gene cluster-linked two-component system that regulates multiple antibiotics in Streptomyces coelicolor. Mol Microbiol 39:553–566
Arias P, Fernandez-Moreno MA, Malpartida F (1999) Characterization of the pathway-specific positive transcriptional regulator for actinorhodin biosynthesis in Streptomyces coelicolor A3(2) as a DNA-binding protein. J Bacteriol 181:6958–6968
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Brian P, Riggle FJ, Santos RA, Champness WC (1996) Global negative regulation of Streptomyces coelicolor antibiotic synthesis mediated by an absA-encoded putative signal transduction system. J Bacteriol 178:3221–3231
Chakraburtty R, White J, Takano E, Bibb M (1996) Cloning, characterization and disruption of a (p)ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2). Mol Microbiol 19:357–368
Chang HM, Chen MY, Shieh YT, Bibb MJ, Chen CW (1996) The cutRS signal transduction system of Streptomyces lividans represses the biosynthesis of the polyketide antibiotic actinorhodin. Mol Microbiol 21:1075–1085
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Fisher SH (1999) Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference!. Mol Microbiol 32:223–232
Fisher SH, Sonenshein AL (1991) Control of carbon and nitrogen-metabolism in Bacillus subtilis. Annu Rev Microbiol 45:107–135
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A 100:1541–1546
Hoch JA (2000) Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3:165–170
Hong HJ, Paget MSB, Buttner MJ (2002) A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Mol Microbiol 44:1199–1211
Horinouchi S, Beppu T (1992) Autoregulatory factors and communication in Actinomycetes. Annu Rev Microbiol 46:377–398
Hutchings MI, Hoskisson PA, Chandra G, Buttner MJ (2004) Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3(2). Microbiology 150:2795–2806
Hutchings MI, Hong HJ, Buttner MJ (2006a) The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol Microbiol 59:923–935
Hutchings MI, Hong HJ, Leibovitz E, Sutcliffe IC, Buttner MJ (2006b) The sigma(E) cell envelope stress response of Streptomyces coelicolor is influenced by a novel lipoprotein, CseA. J Bacteriol 188:7222–7229
Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S (2003) Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol 21:526–531
Ishizuka H, Horinouchi S, Kieser HM, Hopwood DA, Beppu T (1992) A putative 2-component regulatory system involved in secondary metabolism in Streptomyces spp. J Bacteriol 174:7585–7594
Kieser T, Bibb MJ, Buttler MJ, Chater K, Hopwood D (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
Lu YH, Wang WH, Shu D, Zhang WW, Chen L, Qin ZJ, Yang S, Jiang WH (2007) Characterization of a novel two-component regulatory system involved in the regulation of both actinorhodin and a type I polyketide in Streptomyces coelicolor. Appl Microbiol Biotechnol 77:625–635
Ma HT, Kendall K (1994) Cloning and analysis of a gene-cluster from Streptomyces coelicolor that causes accelerated aerial mycelium formation in Streptomyces lividans. J Bacteriol 176:3800–3811
Maheswaran M, Forchhammer K (2003) Carbon-source-dependent nitrogen regulation in Escherichia coli is mediated through glutamine-dependent GlnB signalling. Microbiology 149:2163–2172
McKenzie NL, Nodwell JR (2007) Phosphorylated AbsA2 negatively regulates antibiotic production in Streptomyces coelicolor through interactions with pathway-specific regulatory gene promoters. J Bacteriol 189:5284–5292
Mizuno T (1997) Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res 4:161–168
Mizuno T (2005) Two-component phosphorelay signal transduction systems in plants: from hormone responses to circadian rhythms. Biosci Biotechnol Biochem 69:2263–2276
Ohnishi Y, Ishikawa J, Hara H, Suzuki H, Ikenoya M, Ikeda H, Yamashita A, Hattori M, Horinouchi S (2008) Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J Bacteriol 190:4050–4060
Paget MSB, Chamberlin L, Atrih A, Foster SJ, Buttner MJ (1999a) Evidence that the extracytoplasmic function sigma factor sigma(E) is required for normal cell wall structure in Streptomyces coelicolor A3(2). J Bacteriol 181:204–211
Paget MSB, Leibovitz E, Buttner MJ (1999b) A putative two-component signal transduction system regulates sigma(E), a sigma factor required for normal cell wall integrity in Streptomyces coelicolor A3(2). Mol Microbiol 33:97–107
Parkinson JS, Kofoid EC (1992) Communication Modules in bacterial signaling proteins. Annu Rev Genet 26:71–112
Redenbach M, Kieser HM, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood DA (1996) A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol 21:77–96
Rodriguez-Garcia A, Barreiro C, Santos-Beneit F, Sola-Landa A, Martin JF (2007) Genome-wide transcriptomic and proteomic analysis of the primary response to phosphate limitation in Streptomyces coelicolor M145 and in a Delta phoP mutant. Proteomics 7:2410–2429
Ryding NJ, Anderson TB, Champness WC (2002) Regulation of the Streptomyces coelicolor calcium-dependent antibiotic by absA, encoding a cluster-linked two-component system. J Bacteriol 184:794–805
Sheeler NL, MacMillan SV, Nodwell JR (2005) Biochemical activities of the absA two-component system of Streptomyces coelicolor. J Bacteriol 187:687–696
Sola-Landa A, Moura RS, Martin JF (2003) The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proc Natl Acad Sci U S A 100:6133–6138
Sola-Landa A, Rodriguez-Garcia A, Franco-Dominguez E, Martin JF (2005) Binding of PhoP to promoters of phosphate-regulated genes in Streptomyces coelicolor: identification of PHO boxes. Mol Microbiol 56:1373–1385
Sola-Landa A, Rodriguez-Garci A, Apel AK, Martin JF (2008) Target genes and structure of the direct repeats in the DNA-binding sequences of the response regulator PhoP in Streptomyces coelicolor. Nucleic Acids Res 36:1358–1368
Sun JH, Kelemen GH, Fernandez-Abalos JM, Bibb MJ (1999) Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2). Microbiology 145:2221–2227
Takano E, Gramajo HC, Strauch E, Andres N, White J, Bibb MJ (1992) Transcriptional regulation of the RedD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2). Mol Microbiol 6:2797–2804
Ulrich LE, Koonin EV, Zhulin IB (2005) One-component systems dominate signal transduction in prokaryotes. Trends Microbiol 13:52–56
Umeyama T, Horinouchi S (2001) Autophosphorylation of a bacterial serine/threonine kinase, AfsK, is inhibited by KbpA, an AfsK-binding protein. J Bacteriol 183:5506–5512
Wang WW, Gao J, Chiao JS, Zhao GP, Jiang WH (2004) A novel two-component system amrB-amkB involved in the regulation of central carbohydrate metabolism in rifamycin SV-producing Amycolatopsis mediterranei U32. Curr Microbiol 48:14–19
Wei G, Jiang WH, Yang YL, Chiao JS (2004) Improvement of transformation and electroduction in avermectin high-producer, Streptomyces avermitilis. Folia Microbiol 49:399–405
White J, Bibb M (1997) bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol 179:627–633
Zhang WW, Shi L (2005) Distribution and evolution of multiple-step phosphorelay in prokaryotes: lateral domain recruitment involved in the formation of hybrid-type histidine kinases. Microbiology 151:2159–2173
Acknowledgement
This work was supported by the National Natural Science Foundation of China (30770023, 30700022), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-G-007), and the National Basic Research Program of China (2007CB707803).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Primers used in the construction of deletion mutants and gene overexpression in this study (DOC 40 kb)
Supplementary Table 2
List of primers used in RT-PCR analysis (DOC 44 kb)
Rights and permissions
About this article
Cite this article
Shu, D., Chen, L., Wang, W. et al. afsQ1-Q2-sigQ is a pleiotropic but conditionally required signal transduction system for both secondary metabolism and morphological development in Streptomyces coelicolor . Appl Microbiol Biotechnol 81, 1149–1160 (2009). https://doi.org/10.1007/s00253-008-1738-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1738-1