Abstract
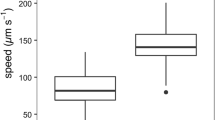
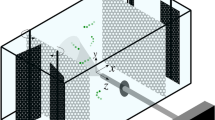
In this study, we used a microchamber to observe and analyze the gliding phenomenon of Navicula pavillardii diatom cells at different temperatures. The temperature of the culture medium was varied from 17.0 to 30.0 °C to examine the effect of temperature on diatom movement. Movement of each cell at different temperatures was monitored by use of an inverted optical microscope and continuously recorded as video data, from which the velocities of each cell were calculated, by using dedicated software to perform two-dimensional trajectory analysis. The velocities of the same cell at different temperatures were thereby successfully compared. The results showed that the change in cell velocity was insignificant when the temperature was increased from 17.0 to 25.0 °C. When the temperature was increased from 17.0 to 27.5 °C, non-uniformly disrupted cell movement was observed. When the temperature was further increased to 30.0 °C, cell movement was clearly inhibited. By use of single-cell analysis, the effects of the temperature increases on diatom movement were successfully evaluated. Finally, we characterized the experimental data by performing t tests to evaluate the effects of variations of the movement of individual cells on the data analysis.


Similar content being viewed by others
References
Apoya-Horton MD, Liang Y, Underwood GJC, Gretz MR (2006) Movement modalities and responses to environmental changes of the mudflat diatom Cylindrotheca closterium (Bacillariophyceae). J Phycol 42:379–390. doi:10.1111/j.1529-8817.2006.00194.x
Armbrust EV (2009) The life of diatoms in the world’s oceans. Nature 459:185–192. doi:10.1038/nature08057
Bowler C, Vardi A, Allen AE (2010) Oceanographic and biogeochemical insights from diatom genomes. Ann Rev Mar Sci 2:333–365. doi:10.1146/annurev-marine-120308-081051
Cartaxana P, Serôdio J (2008) Inhibiting diatom motility: a new tool for the study of the photophysiology of intertidal microphytobenthic biofilms. Limnol Oceanogr Methods 6:466–476. doi:10.4319/lom.2008.6.466
Clarkson N, Davies MS, Dixey R (1999) Diatom motility and low frequency electromagnetic fields: a new technique in the search for independent replication of results. Bioelectromagnetics 20:94–100. doi:10.1002/(SICI)1521-186X(1999)20:2<94::AID-BEM3>3.0.CO;2-Q
Cohn S (2001) Photo-stimulated effects on diatom motility. In: Hader DP, Lebert M (eds) Photomovement. Elsevier, Amsterdam, pp 375–401
Cohn SA, Farrell JF, Munro JD, Ragland RL, Weitzell RE Jr, Wibisono BL (2003) The effect of temperature and mixed species composition on diatom motility and adhesion. Diatom Res 18:225–243
Cohn SA, Bahena M, Davis JT, Ragland RL, Rauschenberg CD, Smith BJ (2004) Characterization of the diatom photophobic response to high irradiance. Diatom Res 19:167–179
Consalvey M, Paterson DM, Underwood GJC (2004) The ups and downs of life in a benthic biofilm: migration of benthic diatoms. Diatom Res 19:181–202
Cooksey B, Cooksey KE (1980) Calcium is necessary for motility in the diatom Amphora coffeaeformis. Plant Physiol 65:129–131. doi:10.1104/pp.65.1.129
Drum RW, Hopkins JT (1966) Diatom locomotion: an explanation. Protoplasma 62:1–33. doi:10.1007/BF01254629
Edgar LA (1979) Diatom locomotion: computer assisted analysis of cine film. Br Phycol J 14:83–101. doi:10.1080/00071617900650111
Edgar LA (1982) Diatom locomotion: a consideration of movement in a highly viscous situation. Br Phycol J 17:243–251. doi:10.1080/00071618200650261
Edgar LA (1983) Mucilage secretions of moving diatoms. Protoplasma 118:44–48. doi:10.1007/BF01284745
Edgar LA, Pickett-Heaps JD (1982) Ultrastructural localization of polysaccharides in the motile diatom Navicula cuspidate. Protoplasma 113:10–22. doi:10.1007/BF01283035
Edgar LA, Pickett-Heaps JD (1983) The mechanism of diatom locomotion. I. An ultrastructural study of the motility apparatus. Proc R Soc Lond B218:331–343. doi:10.1098/rspb.1983.0042
Edgar LA, Pickett-Heaps JD (1984) Diatom locomotion. Prog Phycol Res 3:47–88
Edgar LA, Zavortink M (1983) The mechanism of diatom locomotion. II: identification of Actin. Proc R Soc Lond B218:345–348. doi:10.1098/rspb.1983.0043
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell Science, Malden
Gordon R, Drum RW (1970) A capillarity mechanism for diatom gliding locomotion. Proc Natl Acad Sci USA 67:338–344. doi:10.1073/pnas.67.1.338
Harper MA (1977) The biology of diatoms. In: Werner D (Ed.), Blackwell Scientific Publications, Oxford, pp 224–249. doi:10.1016/0014-4827(72)90416-8
Iwasa K, Shimizu A (1972) Motility of the diatom, Phaeodactylum tricornutum. Exp Cell Res 74:552–558. doi:10.1016/0014-4827(72)90416-8
Lewin JC (1958) The taxonomic position of Phaeodactylum tricornutum. J Gen Microbiol 18:427–432. doi:10.1099/00221287-18-2-427
Moroz AL, Ehrman JM, Clair TA, Gordon RJ, Kaczmarska I (1999) The impact of ultraviolet-B radiation on the motility of the freshwater epipelic diatom Nitzschia lineariz. Global Change Biol 5:191–199. doi:10.1046/j.1365-2486.1999.00225.x
Murase A, Kubota Y, Hirayama S, Kumashiro Y, Okano T, Mayama S, Umemura K (2011) Two-dimensional trajectory analysis of the diatom Navicula sp. using a micro chamber. J Microbiol Methods 87(3):316–319. doi:10.1016/j.mimet.2011.09.006
Murase A, Kubota Y, Hori S, Hirayama S, Mayama S (2012) Importance of observation interval in two-dimensional video analysis of individual diatom cells. Eur Biophys J 41(6):545–550. doi:10.1007/s00249-012-0810-z
Nelson DM, Treguer P, Brzezinski MA, Leynaert A, Queguiner B (1995) Production and dissolution of biogenic silica in the ocean: revised global estimates comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem Cycles 9:359–372. doi:10.1029/95GB01070
Perkins RG, Lavaud J, Serôdio J, Mouget JL, Cartaxana P, Rosa P, Barille L, Brotas V, Jesus BM (2010a) Vertical cell movement is a primary response of intertidal benthic biofilms to increasing light dose. Mar Ecol Progr Ser 416:93–103. doi:10.3354/meps08787
Perkins RG, Kromkamp JC, Serôdio J, Lavaud J, Jesus B, Mouget JL, Lefebvre S, Forster RM (2010) The application of variable chlorophyll fluorescence to microphytobenthic biofilms. In: Suggett DJ et al (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications, developments in applied phycology 4. doi:10.1007/978-90-481-9268-7_12
Pickett-Heaps JD, Hill DRA, Blaze KL (1991) Active gliding motility in an araphid marine diatom, Ardissonea (formerly synedra) crystalline. J Phycol 27:718–725. doi:10.1111/j.0022-3646.1991.00718.x
Poulsen NC, Spector I, Spurck TP, Schultz TF, Wetherbee R (1999) Diatom gliding is the result of an actin–myosin motility system. Cell Motil Cytoskelet 44:23–33. doi:10.1002/(SICI)1097-0169(199909)44:1::23:AID-CM2[3.0.CO;2-D
Umemura K, Haneda T, Tanabe M, Suzuki A, Kumashiro Y, Itoga K, Okano T, Mayama S (2013) Semi-circular micro grooves to observe active movements of individual Navicula pavillardii cells. J Microbiol Meth 92:349–354. doi:10.1016/j.mimet.2013.01.006
Underwood GJC, Kromkamp J (1999) Primary production by phytoplankton and microphytobenthos in estuaries. Adv Ecol Res 29:93–153. doi:10.1016/S0065-2504(08)60192-0
Acknowledgments
This work was supported by RIST TUS Research Center for Green and Safety Sciences.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umemura, K., Miyabayashi, T., Taira, H. et al. Use of a microchamber for analysis of thermal variation of the gliding phenomenon of single Navicula pavillardii cells. Eur Biophys J 44, 113–119 (2015). https://doi.org/10.1007/s00249-015-1006-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-015-1006-0