Abstract
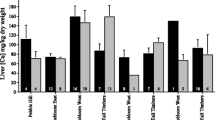
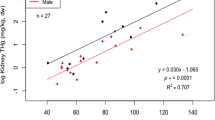
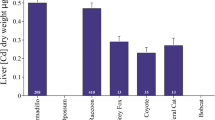
Arsenic is accumulated by free-living small mammals, but there is little information on the resultant concentrations in different tissues other than liver and kidney. Such information is important because the severity of toxicological effects may be related to the amount of arsenic accumulated in specific organs, and the availability of arsenic to predators is, in part, dependent on which tissues accumulate arsenic. The objective of this study was to quantify the arsenic concentrations and the percentage of the total body burden (%TBB) accumulated in different body tissues of free-living small mammals and to determine how these factors varied with severity of habitat contamination. Arsenic concentrations were measured in various tissues of wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) from a range of arsenic-contaminated sites in southwest Britain. Arsenic concentrations in the gastrointestinal (GI) tract (including contents), liver, kidneys, spleen, lung, femur, and fur of both species varied significantly between sites and were higher in mice and voles from heavily contaminated areas. Heart and brain arsenic concentrations did not vary with degree of environmental contamination. The GI tract and excised carcass contained roughly equal amounts of arsenic and, in sum, comprised 75–85% of the TBB on uncontaminated sites and 90–99% on contaminated sites. Although the excised carcass contains about half of the TBB, its importance in food-chain transfer of arsenic to predators may depend on the bioavailability of arsenic sequestered in fur. In contrast, the GI tract and its contents, provided that it is consumed, will always be a major transfer pathway for arsenic to predators, regardless of the severity of habitat contamination.
Similar content being viewed by others
References
Abrahams PW, Thornton I (1987) Distribution and extent of land contaminated by arsenic and associated metals in mining regions of southwest England. Tran Inst Min Metall Sect B-App Earth Sci 96:B1–B8
Abrahams PW, Thornton I (1994) The contamination of agricultural land in the metalliferous province of southwest England—implications to livestock. Agric Ecosyst Environ 48:125–137
Benson LM, Porter EK, Peterson PJ (1981) Arsenic accumulation, tolerance and genotypic variation in plants on arsenical mine wastes in SW England. J Plant Nutr 3:655–666
Beyer WN, Connor EE, Gerould S (1994) Estimates of soil ingestion by wildlife. J Wildl Manage 58:375–382
Blakley BR, Sisodia CS, Mukkur TK (1980) The effect of methylmercury, tetraethyl lead, and sodium arsenite on the humoral immune response in mice. Toxicol Appl Pharmacol 52:245–254
Burger J, Gaines KF, Lord CG, Brisbin IL, Shukla S, Gochfeld M (2002) Metal levels in raccoon tissues: Differences on and off the Department of Energy’s Savannah River site in South Carolina. Environ Monit Assess 74:67–84
Carlson RM (1990) Assessment of the propensity for covalent binding of electrophiles to biological substrates. Environ Health Perspect 87:227–232
Curry AS, Pounds CA (1979) Arsenic in hair. J Forensic Sci Soc 17:37–44
Davies BE (1971) Trace metal content of soils affected by base metal mining in the West of England. Oikos 22:366–372
Davies BE (1983) Heavy metal contamination from base metal mining and smelting: implications for man and his environment. In: Thornton I (ed) Applied environmental geochemistry. Academic Press, London, pp 425–460
Erry BV (1999) Transfer of arsenic through terrestrial foodchains. PhD thesis, University of Exeter
Erry BV, Macnair MR, Meharg AA, Shore RF (1999) Seasonal variation in dietary and body organ arsenic concentrations in wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus). Bull Environ Contam Toxicol 63:567–574
Erry BV, Macnair MR, Meharg AA, Shore RF (2000) Arsenic contamination in wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) on abandoned mine sites in southwest Britain. Environ Pollut 110:179–187
Farago ME, Kavanagh PJ, Leite MJ, Mossom J, Sawbridge G, Thornton I (2002) Uptake of arsenic by plants in southwest England. In: Cai Y, Braids OC (eds) Biogeochemistry of environmentally important trace elements. ACS Symposium Series 835, American Chemical Society, Washington DC, pp 115–127
Flowerdew JR 1985) The population dynamics of wood mice and yellow-necked mice. Symp Zoo Soc Lond 55:315–338
Griffin JL, Walker L, Shore RF, Nicholson JK (2001) High-resolution magic angle spinning H-1-NMR spectroscopy studies on the renal biochemistry in the bank vole (Clethrionomys glareolus) and the effects of arsenic (As3+) toxicity. Xenobiotica 31:377–385
Ismail A, Roberts RD (1992) Arsenic in small mammals. Environ Technol 13:1091–1095
Kavanagh PJ, Farago ME, Thornton I, Braman RS (1997) Bioavailability of arsenic in soil and mine wastes of the Tamar Valley, SW England. Chem Speciation Bioavail 9:77–81
Kollmer WE (1992) Arsenic in induced hair of the rat and its relation to the content in various organs during chronic exposure. J Trace Elements Electrolytes Health Dis 6:11–14
Langdon CJ, Meharg AA, Feldmann J, Balgar T, Charnock J, Farquhar M, Piearce TG, Semple KT, Cotter-Howells J (2002) Arsenic-speciation in arsenate-resistant and non-resistant populations of the earthworm, Lumbricus rubellus. J Environ Monit 4:603–608
Macnair MR (2003) The hyperaccumulation of metals by plants. Adv Bot Res 40: 63–105
Mankovska B, Steinnes E (1995) Effects of pollutants from an aluminum reduction plant on forest ecosystems. Sci Total Environ 163:11–23
Marafante E, Rade J, Sabbioni E, Bertolero F, Foa V (1981) Intracellular interaction and metabolic fate of arsenite in the rabbit. Clin Toxicol 18:1335–1341
Meharg AA, Naylor J, Macnair MR (1994) Phosphorus nutrition of arsenate-tolerant and non-tolerant phenotypes of velvet grass. J Environ Qual 23:234–238
Milton A, Johnson M (1999) Arsenic in the food chains of a revegetated metalliferous mine tailings pond. Chemosphere 39:765–779
Peles JD, Barrett GW (1997) Assessment of metal uptake and genetic damage in small mammals inhabiting a fly ash basin. Bull Environ Contam Toxicol 59:279–284
Peterson PJ, Benson LM, Porter EK (1979) Biogeochemistry of arsenic on polluted sites in SW England. In: Proceedings of an international conference on management and control of heavy metals in the environment. CEP Consultants Ltd, Edinburgh, pp 198–201
Porter EK, Peterson PJ (1975) Arsenic accumulation by plants on mine waste (United Kingdom). Sci Total Environ 4:365–371
Pounds CA, Pearson EF, Turner TD (1979) Arsenic in fingernails. J Forensic Sci Soc 19:165–173
Sample BE, Suter GW (2002) Screening evaluation of the ecological risks to terrestrial wildlife associated with a coal ash disposal site. Hum Ecol Risk Assess 8:637–656
Sharma RP, Shupe JL (1977) Lead, cadmium and arsenic residues in animal tissues in relation to those in their surrounding habitat. Sci Total Environ 7:53–62
Sheffield SR, Sawicka-Kapusta K, Cohen JB, Rattner BA (2001) Rodentia and lagomorpha. In: Shore RF, Rattner BA (ed) Ecotoxicology of wild mammals. John Wiley & Sons, Chichester, UK, pp 215–314
Shore RF, Balment RJ, Yalden DW (1992) The effect of varying calcium intake on calcium metabolism in wild rodent species. J Zool (Lond) 227:29–42
Tersago K, De Coen W, Scheirs J, Vermeulen K, Blust R, Van Bockstaele D, Verhagen R (2004) Immunotoxicology in wood mice along a heavy metal pollution gradient. Environ Pollut 132:385–394
Toal ME, Walker LA, Shore RF (2002) Modelling cadmium dynamics in the guts and tissues of small mammals: dose implications for higher predators. Environ Toxicol Chem 21:2493–2499
Torres KC, Johnson ML (2001) Bioaccumulation of metals in plants, arthropods, and mice at a seasonal wetland. Environ Toxicol Chem 20:2617–2626
Vahter M (1981) Biotransformation of trivalent and pentavalent inorganic arsenic in mice and rats. Environ Res 25:286–293
Vahter M (1983) Metabolism of arsenic. In: Fowler B (ed) Biological and environmental effects of arsenic. Elsevier, London, pp 171–198
Vahter M 1994a) Species differences in the metabolism of arsenic compounds. Appl Organomet Chem 8:175–182
Vahter M 1994b) What are the chemical forms of arsenic in urine, and what can they tell us about exposure. Clin Chem 40:679–680
Vahter M, Marafante E (1979) In vivo methylation and detoxication of arsenic. In: The biological alkylation of heavy elements. Royal Society of Chemistry, London, pp 105–119
Vahter M, Norin H (1980) Metabolism of 74-As labeled trivalent and pentavalent inorganic arsenic in mice. Environ Res 21:446–457
Vahter M, Marafante E (1983) Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chem-Biol Interact 47:29–44
Vahter M, Marafante E, Dencker L (1984) Tissue distribution and retention of As-74 dimethylarsinic acid in mice and rats. Arch Environ Contam Toxicol 13:259–264
Walker LA, Bailey LJ, Shore RF (2002) The importance of the gut and its contents in prey as a source of cadmium to predators. Environ Toxicol Chem 21:76–80
WHO (2001) Environmental health criteria 224: Arsenic and arsenic compounds, second edition. WHO, Geneva
Wilhelm M, Idel H (1996) Hair analysis in environmental medicine. Zent bl Hyg Umweltmed 198:485–501
Yamauchi H, Yamamura Y (1985) Metabolism and excretion of orally administrated arsenic trioxide in the hamster. Toxicology 34:113–121
Acknowledgments
This work was made possible through funding from NERC grant no. GT/104/95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erry, B.V., Macnair, M.R., Meharg, A.A. et al. The Distribution of Arsenic in the Body Tissues of Wood Mice and Bank Voles. Arch Environ Contam Toxicol 49, 569–576 (2005). https://doi.org/10.1007/s00244-004-0229-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0229-3