Abstract
Objectives
To summarize the pharmacokinetics of linezolid to optimize the dosing regimen in special populations.
Methods
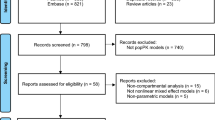
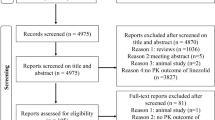
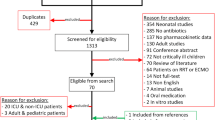
A literature search was performed in three largest medical databases, including Embase, Scopus, and PubMed. The main applied keywords were linezolid and pharmacokinetics. Of 3663 retrieved publications in the English language, 35 original research articles, clinical studies, and case reports about linezolid pharmacokinetics in different populations such as pregnant women, pediatrics, elderly subjects, obese people, individuals with organ dysfunction, and critically ill patients were included.
Results and conclusion
Dose adjustment is not currently recommended for linezolid in patients with mild to moderate renal or hepatic impairment, older adults, and pregnant women. Although dose adjustment is not recommended in patients with severe renal or hepatic impairment, it should be considered that these patients are more vulnerable to linezolid adverse effects and drug interactions. In pediatrics, reducing the linezolid dosing interval to 8 h is suggested. Despite the lack of sufficient information in obese individuals, dosing based on body weight or use of higher dose seems to be justifiable to prevent sub-therapeutic concentrations. Although dose adjustment of linezolid is not recommended in critically ill patients, administration of linezolid as continuous intravenous infusion is suggested in this population. Blood level monitoring should be considered in populations that are vulnerable to linezolid underexposure (such as critically ill patients with augmented renal clearance, pediatrics, overweight, and obese patients) or overexposure (such as elderly, patients with hepatic and renal impairment). To assess the efficacy and safety of linezolid, the area under the concentration–time curve over 24 h to minimum inhibitory concentration (AUC0–24 h/MIC) equal to 80–120, percentage of time above the MIC ≥ 85%, and serum trough concentration between 2 and 7 mg/L are suggested.
Similar content being viewed by others
Data availability
Available per request.
References
Zahedi Bialvaei A, Rahbar M, Yousefi M, Asgharzadeh M, Samadi Kafil H (2017) Linezolid: a promising option in the treatment of gram-positives. J Antimicrob Chemother 72:354–364. https://doi.org/10.1093/jac/dkw450
Welshman I, Stalker D, Wajszczuk C (1998) Assessment of absolute bioavailability and evaluation of the effect of food on oral bioavailability of linezolid. Antiinfect Drugs Chemother 16(Suppl 1):54
Dryden MS (2011) Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother 66 Suppl 4:iv7–iv15. https://doi.org/10.1093/jac/dkr072
Sazdanovic P, Jankovic SM, Kostic M, Dimitrijevic A, Stefanovic S (2016) Pharmacokinetics of linezolid in critically ill patients. Expert Opin Drug Metab Toxicol 12:595–600. https://doi.org/10.1517/17425255.2016.1170807
Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O’Grady M, Hopkins NK, Jungbluth GL (2003) Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother 47:2775–2780. https://doi.org/10.1128/AAC.47.9.2775-2780.2003
Sisson TL, Jungbluth GL, Hopkins NK (2002) Age and sex effects on the pharmacokinetics of linezolid. Eur J Clin Pharmacol 57:793–797. https://doi.org/10.1007/s00228-001-0380-y
Cai Y, Chai D, Falagas ME, Karageorgopoulos DE, Wang R, Bai N, Liang B (2013) Weight-adjusted versus fixed dose of linezolid for Chinese healthy volunteers of higher and lower body weight: a phase I pharmacokinetic and pharmacodynamic study. Expert OpinInvestig Drugs 22:309–315. https://doi.org/10.1517/13543784.2013.766716
Jungbluth GL, Welshman IR, Hopkins NK (2003) Linezolid pharmacokinetics in pediatric patients: an overview. Pediatr Infect Dis J 22(9 Suppl):S153–S157. https://doi.org/10.1097/01.inf.0000086954
Bhalodi AA, Papasavas PK, Tishler DS, Nicolau DP, Kuti JL (2013) Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob Agents Chemother 57:1144–1149. https://doi.org/10.1128/AAC.01453-12
Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M (2012) Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 67:2034–2042. https://doi.org/10.1093/jac/dks153
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, Johnson MG, Sanders PE, Hauer MJ, Fagerness PE, Stryd RP, Peng GW, Shobe EM (2001) Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C] linezolid to healthy human subjects. Drug MetabDispos 29(8):1136–1145
MacGowan AP (2003) Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with gram-positive infections. J Antimicrob Chemother51 Suppl 2:ii17–25. https://doi.org/10.1093/jac/dkg248.
Stalker DJ, Jungbluth GL (2003) Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinetic 42:1129–1140. https://doi.org/10.2165/00003088-200342130-00004
Pea F, Cojutti PG, Baraldo M (2017) A 10-year experience of therapeutic drug monitoring (TDM) of linezolid in a hospital-wide population of patients receiving conventional dosing: is there enough evidence for suggesting TDM in the majority of patients? Basic Clin Pharmacol Toxicol 121:303–308. https://doi.org/10.1111/bcpt.12797
Cattaneo D, Alffenaar JW, Neely M (2016) Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin Drug Metab Toxicol 12:533–44. https://doi.org/10.1517/17425255.2016.1166204
Sasaki T, Takane H, Ogawa K, Isagawa S, Hirota T, Higuchi S, Horii T, Otsubo K, Ieiri I (2011) Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob Agents Chemother 55:1867–1873. https://doi.org/10.1128/AAC.01185-10
Abdul-Aziz MH, Alffenaar J, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely M et al (2020) Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper infection section of European Society of Intensive Care Medicine (ESICM); Pharmacokinetic/pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID); Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT); Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC). Intensive Care Med 46:1127–1153
Rao GG, Konicki R, Cattaneo D, Alffenaar JW, Marriott DJE, Neely M (2020) IATDMCT Antimicrobial Scientific Committee. Therapeutic drug monitoring can improve linezolid dosing regimens in current clinical practice: a review of linezolid pharmacokinetics and pharmacodynamics. Ther Drug Monit 42:83–92. https://doi.org/10.1097/FTD.0000000000000710
Rayner CR, Forrest A, Meagher A, Birmingham M, Schentag J (2003) Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet 42:1411–1423
Andes D, Ogtrop M, Peng J, Craig W (2002) In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 46:3484–3489
Wienkers L, Wynalda M, Feenstra K, Gao P, Slatter J, editors (1999) In vitro metabolism of linezolid (PNU-100766): lack of induction or inhibition of cytochrome P450 enzymes and studies on the mechanism of formation of the major human metabolite, PNU-142586. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco (CA)
Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB (2011) Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genomics 21:152–161. https://doi.org/10.1097/FPC.0b013e3283385a1c
Leschziner GD, Andrew T, Pirmohamed M, Johnson MR (2007) ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J 7:154–179. https://doi.org/10.1038/sj.tpj.6500413
Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, Morikawa N, Takeda Y (2015) Linezolid dosage in pediatric patients based on pharmacokinetics and pharmacodynamics. J Infect Chemother 21:70–73. https://doi.org/10.1016/j.jiac.2014.08.017
Cojutti P, Maximova N, Crichiutti G, Isola M, Pea F (2015) Pharmacokinetic/pharmacodynamic evaluation of linezolid in hospitalized paediatric patients: a step toward dose optimization by means of therapeutic drug monitoring and Monte Carlo simulation. J Antimicrob Chemother 70:198–206. https://doi.org/10.1093/jac/dku337
Li SC, Ye Q, Xu H, Zhang L, Wang Y (2019) Population pharmacokinetics and dosing optimization of linezolid in pediatric patients. Antimicrob Agents Chemother 27(63):e02387-e2418. https://doi.org/10.1128/AAC.02387-18
Tinelli M, Gervasoni C, Piazza M, Terzi R, Cozzi V, Maffezzini E, Cerri C, Castoldi S, Baldelli S, Cattaneo D (2017) Is it time to revise linezolid dose in elderly patients? Eur J Clin Pharmacol 73:1335–1336. https://doi.org/10.1007/s00228-017-2303-6
Cattaneo D, Fusi M, Cozzi V, Baldelli S, Bonini I, Gervasoni C, Clementi E (2021) Supra-therapeutic linezolid trough concentrations in elderly patients: a call for action? Clin Pharmacokinet 60:603–609. https://doi.org/10.1007/s40262-020-00964-1
Muzevich KM, Lee KB (2013) Subtherapeutic linezolid concentrations in a patient with morbid obesity and methicillin-resistant Staphylococcus aureus pneumonia: case report and review of the literature. Ann Pharmacother 47:e25. https://doi.org/10.1345/aph.1R707
Cojutti P, Pai MP, Pea F (2018) Population pharmacokinetics and dosing considerations for the use of linezolid in overweight and obese adult patients. Clin Pharmacokinet 57:989–1000. https://doi.org/10.1007/s40262-017-0606-5
**e F, Mantzarlis K, Malliotakis P, Koulouras V, Degroote S, Koulenti D, Blot S, Boussery K, Van Bocxlaer J, Colin P (2019) LIMOP study collaborators. Pharmacokinetic evaluation of linezolid administered intravenously in obese patients with pneumonia. J Antimicrob Chemother74:667–674. https://doi.org/10.1093/jac/dky500
Blackman AL, Jarugula P, Nicolau DP, Chui SH, Joshi M, Heil EL, Gopalakrishnan M (2021) Evaluation of linezolid pharmacokinetics in critically ill obese patients with severe skin and soft tissue infections. Antimicrob Agents Chemother J 65(2):e01619–20. https://doi.org/10.1128/AAC.01619-20
L Ehmann L, Simon P, Busse D, Petroff D, Dorn C, Huisinga W, Dietrich A et al (2020) Risk of target non-attainment in obese compared to non-obese patients in calculated linezolid therapy. Clin Microbiol Infect 26(9):1222–1228. Epub 2020 Apr 18. PMID: 32311473. https://doi.org/10.1016/j.cmi.2020.04.009
Costantine MM (2014) Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 5:65. https://doi.org/10.3389/fphar.2014.00065
Loebstein R, Lalkin A, Koren G (1997) Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet 33(5):328–343. https://doi.org/10.2165/00003088-199733050-00002
Tasnif Y, Morado J, Hebert MF (2016) Pregnancy-related pharmacokinetic changes. Clin Pharmacol Ther 100(1):53–62. https://doi.org/10.1002/cpt.382
Van Kampenhout E, Bolhuis MS, Alffenaar JC, Oswald LM, Kerstjens HA, de Lange WC, van der Werf TS, Akkerman OW (2017) Pharmacokinetics of moxifloxacin and linezolid during and after pregnancy in a patient with multidrug-resistant tuberculosis. Eur Respir J 49(3):1601724. https://doi.org/10.1183/13993003.01724-2016
Takahashi Y, Takesue Y, Nakajima K, Ichiki K, Tsuchida T, Tatsumi T et al (2011) Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J Infect Chemother 17:382–387
Luque S, Muñoz-Bermudez R, Echeverría-Esnal D, Sorli L, Campillo N, Martínez-Casanova J, González-Colominas E, Álvarez-Lerma F, Horcajada JP, Grau S, Roberts JA (2019) Linezolid dosing in patients with liver cirrhosis: standard dosing risk toxicity. Ther Drug Monit 41:732–739. https://doi.org/10.1097/FTD.0000000000000665
Zhang SH, Zhu ZY, Chen Z, Li Y, Zou Y, Yan M, Xu Y, Wang F, Liu MZ, Zhang M, Zhang BK (2020) Population pharmacokinetics and dosage optimization of linezolid in patients with liver dysfunction. Antimicrob Agents Chemother 64(6):e00133-e220. https://doi.org/10.1128/AAC.00133-20
Crass RL, Cojutti P, Pai M, Pea F, Crass RL, Cojutti PG, Pai MP, Pea F (2019) Reappraisal of linezolid dosing in renal impairment to improve safety. Antimicrob Agents Chemother 63(8):e00605–19. https://doi.org/10.1128/AAC.00605-19
Kawasuji H, Tsuji Y, Ogami C, Kimoto K, Ueno A, Miyajima Y, Kawago K, Sakamaki I, Yamamoto Y (2021) Proposal of initial and maintenance dosing regimens with linezolid for renal impairment patients. BMC Pharmacol Toxicol 22(1):13. https://doi.org/10.1186/s40360-021-00479-w
Shi C, **a J, Ye J, **e Y, ** W, Zhang W, Wang L, Ding X, Lin N, Wang L (2022) Effect of renal function on the risk of thrombocytopaenia in patients receiving linezolid therapy: a systematic review and meta-analysis. Br J Clin Pharmacol 88(2):464–475. https://doi.org/10.1111/bcp.14965
Bolhuis MS, Akkerman O, Sturkenboom M, Ghimire S, Srivastava S, Gumbo T, Alffenaar J et al (2018) Linezolid-based regimens for multidrug-resistant tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis 67:S327-s335
Millard J, Pertinez H, Bonnett L, Hodel E, Dartois V, L Johnson J, et al (2018) Linezolid pharmacokinetics in MDR-TB: a systematic review, meta-analysis and Monte Carlo simulation. J Antimicrob Chemother 73:1755–1762
Alghamdi WA, Al-Shaer M, An G, Alsultan A, Kipiani M, Barbakadze K et al (2020) Population pharmacokinetics of linezolid in tuberculosis patients: dosing regimen simulation and target attainment analysis. Antimicrob Agents Chemother 64:e01174-e1220
Tietjen AK, Kroemer N, Cattaneo D, Baldelli S, Wicha S (2022) Population pharmacokinetics and target attainment analysis of linezolid in multidrug-resistant tuberculosis patients. Br JClin Pharmacol 88:1835–1844
De PJ, Sutter Gasthuys PJ, Van Braeckel E, Schelstraete P, Van Biervliet S, Van Bocxlaer J, Vermeulen A (2020) Pharmacokinetics in patients with cystic fibrosis: a systematic review of data published between 1999 and 2019. Clin Pharmacokinet. 2020 Dec 59(12):1551–1573. PMID: 32808233. https://doi.org/10.1007/s40262-020-00932-9
Magréault S, Roy S, Launay C, Sermet-Gaudelus M, Jullien IV (2021) Pharmacokinetic and pharmacodynamic optimization of antibiotic therapy in cystic fibrosis patients: current evidences, gaps in knowledge and future directions. Clin Pharmacokinet. Apr;60(4):409–445. Epub 2021 Jan 24. PMID: 33486720. https://doi.org/10.1007/s40262-020-00981-0
Bosso JA, Flume PA, Gray SL (2004) Jan Linezolid pharmacokinetics in adult patients with cystic fibrosis Antimicrob Agents Chemother 48(1):281–284. https://doi.org/10.1128/AAC.48.1.281-284.2004. PMID:14693551; PMCID:PMC310170
Santos RP, Prestidge CB, Brown ME, Urbancyzk B, Murphey DK, Salvatore CM et al (2009) Feb Pharmacokinetics and pharmacodynamics of linezolid in children with cystic fibrosis Pediatr Pulmonol 44(2):148–154. PMID: 19137597. https://doi.org/10.1002/ppul.20966
Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, Nicolau DP, Kuti JL (2011) Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrob Agents Chemother 55(7):3393–8. Epub 2011 Apr 25. PMID: 21518837; PMCID: PMC3122391. https://doi.org/10.1128/AAC.01797-10
Blot SI, Pea F, Lipman J (2014) The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. https://doi.org/10.1016/j.addr.2014.07.006
Felton TW, Hope WW, Roberts JA (2014) How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis 79:441–447. https://doi.org/10.1016/j.diagmicrobio.2014.04.007
Roberts JA, Lipman J (2009) Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–51. quiz 859. https://doi.org/10.1097/CCM.0b013e3181961bff
Sun Z, Zhang X, Zheng J, Hou G, Han Q, Qi Z, Huang R, Yu Y, Liu G, Gao Y, Ye M, Li M, Yu K, Wang HL (2020) Pharmacokinetics and Pharmacodynamics of linezolid following intragastric and intravenous administrations in ICU patients. Preprint in Research Square. https://doi.org/10.21203/rs.3.rs-102976/v1
Beringer P, Nguyen M, Hoem N, Louie S, Gill M, Gurevitch M, Wong-Beringer A (2005) Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings. Antimicrob Agents Chemother 49:3676–3681. https://doi.org/10.1128/AAC.49.9.3676-3681
Vanstraelen K, Wauters J, Vercammen I, de Loor H, Maertens J, Lagrou K, Annaert P, Spriet I (2014) Impact of hypoalbuminemia on voriconazole pharmacokinetics in critically ill adult patients. Antimicrob Agents Chemother 58:6782–6789. https://doi.org/10.1128/AAC.03641-14
Plock N, Buerger C, Joukhadar C, Kljucar S, Kloft C (2007) Does linezolid inhibit its own metabolism? Population pharmacokinetics as a tool to explain the observed nonlinearity in both healthy volunteers and septic patients. Drug Metab Dispos 35:1816–23. https://doi.org/10.1124/dmd.106.013755
Barrasa H, Soraluce A, Usón E, Sainz J, Martín A, Sánchez-Izquierdo JÁ, Maynar J, Rodríguez-Gascón A, Isla A (2020) Impact of augmented renal clearance on the pharmacokinetics of linezolid: advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int J Infect Dis 93:329–338. https://doi.org/10.1016/j.ijid.2020.02.044
Wang X, Wang Y, Yao F, Chen S, Hou Y, Zheng Z, Luo J, Qiu B, Li Z, Wang Y, Wu Z, Lan J, Chen C (2021) Pharmacokinetics of linezolid dose adjustment for creatinine clearance in critically ill patients: a multicenter, prospective, open-label, observational study. Drug Des Devel Ther 15:2129–2141. https://doi.org/10.2147/DDDT.S303497
Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Parenti E, Picetti E, Sagripanti S, Manini P, Andreoli R, Cabassi A (2004) Removal of linezolid by conventional intermittent hemodialysis, sustained low-efficiency dialysis, or continuous venovenous hemofiltration in patients with acute renal failure. Crit Care Med 32:2437–2442. https://doi.org/10.1097/01.ccm.0000147687.06808.92
El-Assal MI, Helmy SA (2014) Single-dose linezolid pharmacokinetics in critically ill patients with impaired renal function especially chronic hemodialysis patients. Biopharm Drug Dispos 35:405–416. https://doi.org/10.1002/bdd.1910
Barrasa H, Soraluce A, Isla A, Martín A, Maynar J, Canut A, Sánchez-Izquierdo JA, Rodríguez-Gascón A (2019) Pharmacokinetics of linezolid in critically ill patients on continuous renal replacement therapy: influence of residual renal function on PK/PD target attainment. J Crit Care 50:69–76. https://doi.org/10.1016/j.jcrc.2018.11.016
Ha MA, Sieg AC (2017) Evaluation of altered drug pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation. Pharmacotherapy 37:221–235. https://doi.org/10.1002/phar.1882
Shekar K, Roberts JA, Mcdonald CI, Ghassabian S, Anstey C, Wallis SC, Mullany DV, Fung YL, Fraser JF (2015) Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care 19(1):164. https://doi.org/10.1186/s13054-015-0891-z
Nikolos P, Osorio J, Mohrien K, Rose C (2020) Pharmacokinetics of linezolid for methicillin-resistant Staphylococcus aureus pneumonia in an adult receiving extracorporeal membrane oxygenation. Am J Health Syst Pharm 77:877–881. https://doi.org/10.1093/ajhp/zxaa066
De Rosa FG, Corcione S, Baietto L, Ariaudo A, Di Perri G, Ranieri VM, D’Avolio A (2013) Pharmacokinetics of linezolid during extracorporeal membrane oxygenation. Int J Antimicrob Agents 41:590–591. https://doi.org/10.1016/j.ijantimicag.2013.01.016
Adembri C, Fallani S, Cassetta MI, Arrigucci S, Ottaviano A, Pecile P, Mazzei T, De Gaudio R, Novelli A (2008) Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int J Antimicrob Agents 31:122–129. https://doi.org/10.1016/j.ijantimicag.2007.09.009
Soraluce A, Barrasa H, Asín-Prieto E, Sánchez-Izquierdo JÁ, Maynar J, Isla A, Rodríguez-Gascón A (2020) Novel population pharmacokinetic model for linezolid in critically ill patients and evaluation of the adequacy of the current dosing recommendation. Pharmaceutics 12:54. https://doi.org/10.3390/pharmaceutics12010054
Douros A, Grabowski K, Stahlmann R (2015) Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin Drug Metab Toxicol 11:1849–59. https://doi.org/10.1517/17425255.2015.1098617
Acknowledgements
Dr. Abidi Pharmaceutical Company supported this work
Author information
Authors and Affiliations
Contributions
Shima Heidari: literature review, selecting articles, and drafting the manuscript. Hossein Khalili: conceptualization, analysis, and editing and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heidari, S., Khalili, H. Linezolid pharmacokinetics: a systematic review for the best clinical practice. Eur J Clin Pharmacol 79, 195–206 (2023). https://doi.org/10.1007/s00228-022-03446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03446-4