Abstract
Purpose
Data from two clinical studies (hyperCholesterolaemia in cHildren and Adolescents taking Rosuvastatin OpeN label [CHARON; NCT01078675] and Study 4522IL/0086) were used to describe rosuvastatin pharmacokinetics in patients with heterozygous familial hypercholesterolemia aged ≥6 to <18 years.
Methods
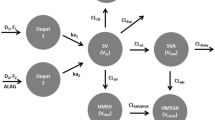
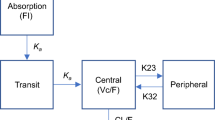
Rosuvastatin concentration–time data were analyzed via non-linear mixed-effects modeling (NONMEM), with clearance (CL/F) as the pre-defined key pharmacokinetic parameter of interest. In addition, descriptive comparisons between pediatric patients and adults (healthy and dyslipidemic) were performed. The dataset included 214 pediatric patients, with 2,029 rosuvastatin concentrations.
Results
A linear two-compartment model with first-order absorption and elimination processes adequately described the combined dataset. Weight and gender were significant covariates for CL/F, with moderate between-patient variability remaining (coefficient of variation (CV) 40 %): CL/F in female children was approximately 30 % lower than in male children, and there was a twofold mean difference in CL/F across the observed weight range. Age was not a significant covariate after accounting for weight and gender differences. However, weight and gender only reduced between-patient variability from 45 (without covariates) to 40 % and are considered unlikely to be clinically relevant.
Conclusions
Rosuvastatin pharmacokinetics appeared generally predictable with respect to dose, and time (study duration) and the exposure (dose-normalized area under the plasma concentration–time curve at steady state (AUCss)) in children and adolescents appeared to be similar or lower than adult patients with dyslipidemia.




Similar content being viewed by others
References
Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ (2010) Meta-analysis of comparative efficacy of increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol 105:69–76
Tzeng TB, Schneck DW, Birmingham BK, Mitchell PD, Zhang H, Martin PD, Kung LP (2008) Population pharmacokinetics of rosuvastatin: implications of renal impairment, race, and dyslipidaemia. Curr Med Res Opin 24:2575–2585
Igel M, Sudhop T, von Bergmann K (2002) Pharmacology of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), including rosuvastatin and pitavastatin. J Clin Pharmacol 42:835–845
Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, Lenz E (2003) Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther 25:2822–2835
Luvai A, Mbagaya W, Hall AS, Barth JH (2012) Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol 6:17–33
AstraZeneca. CRESTOR SmPC http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Crestor_29/WC500010495.pdf. Accessed 15 March 2014
Martin PD, Dane AL, Nwose OM, Schneck DW, Warwick MJ (2002) No effect of age or gender on the pharmacokinetics of rosuvastatin: a new HMG-CoA reductase inhibitor. J Clin Pharmacol 42:1116–1121
Avis HJ, Hutten BA, Gagne C, Langslet G, McCrindle BW, Wiegman A, Hsia J, Kastelein JJ, Stein EA (2010) Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J Am Coll Cardiol 55:1121–1126
Langslet G, Braamskamp MJ, McCrindle B, Cassiman D, Francis G, Gagne C, Gaudet D, Morrison KM, Wiegman A, Turner T, Miller E, Raichlen J, Martin PG, Stein E, Kastelein J (2014) Effect of rosuvastatin therapy on arterial wall changes in children and adolescents with familial hypercholesterolemia: results from the CHARON study. J Am Coll Cardiol 63(Suppl 1):A1379
AstraZeneca. Crestor (Rosuvastatin calcium) tablets. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/Develop%20mentResources/UCM205895.pdf. Accessed 5 February 2015
Hull CK, Penman AD, Smith CK, Martin PD (2002) Quantification of rosuvastatin in human plasma by automated solid-phase extraction using tandem mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci 772:219–228
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM 7.2.0 users guides. Ellicott City, Maryland, USA: Icon Development Solutions; 1989–2011. Introduction to NONMEM 7.2.0. https://nonmem.iconplc.com/nonmem7/Release_Notes_Plus/nm720.pdf. Accessed 20 August 2015
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 96:1336–1345
Van Kesteren C, Mathot RA, Raymond E, Armand JP, Dittrich C, Dumez H, Roche H, Droz JP, Punt C, Ravic M, Wanders J, Beijnen JH, Fumoleau P, Schellens JH (2002) Population pharmacokinetics of the novel anticancer agent E7070 during four phase I studies: model building and validation. J Clin Oncol 20:4065–4073
European Medicines Agency Committee for Medicinal Products in Human Use. Guideline on the role of pharmacokinetics in the development of medicinal products in the paediatric population http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003066.pdf. Accessed 5 February 2015
Edginton AN, Shah B, Sevestre M, Momper JD (2013) The integration of allometry and virtual populations to predict clearance and clearance variability in pediatric populations over the age of 6 years. Clin Pharmacokinet 52:693–703
Acknowledgments
This study was supported by AstraZeneca. Medical writing support was provided by Melanie More of Prime Medica Ltd, Knutsford, Cheshire, UK, funded by AstraZeneca. Carl-Christer Johansson and Daniel Röshammar of AstraZeneca assisted with analysis of the data.
Author contributions
M. Macpherson: data analysis and interpretation and drafting the manuscript.
P.D. Martin: data analysis and interpretation.
B. Hamrén: data analysis and interpretation and drafting the manuscript.
T. Lundström: data analysis and interpretation.
J.J.P. Kastelein: data collection, data analysis, and interpretation.
M.J.A.M. Braamskamp: data collection, data analysis, and interpretation.
All authors revised the manuscript critically for important intellectual content at each stage of development, approved the final version of the manuscript for publication, and agreed to be accountable for all aspects of the work.
Conflict of interest
P.D. Martin is an employee of AstraZeneca, Macclesfield, UK. M. Macpherson is a former employee of AstraZeneca, Macclesfield, UK. B. Hamrén and T. Lundström are employees of AstraZeneca, Mölndal, Sweden. P.D. Martin, M. Macpherson, and B. Hamrén are shareholders of AstraZeneca. J.J.P. Kastelein has received grant support from AstraZeneca, Pfizer, Roche, Novartis, Merck, Merck/Schering-Plough, Isis, Genzyme, and Sanofi-Aventis; lecture fees from AstraZeneca, GlaxoSmithKline, Pfizer, Novartis, Merck/Schering-Plough, Roche, Isis, and Boehringer Ingelheim; and consulting fees from AstraZeneca, Abbott, Pfizer, Isis, Genzyme, Roche, Novartis, Merck, Merck/Schering-Plough, and Sanofi-Aventis. M.J.A.M. Braamskamp has nothing to declare.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1,565 kb)
Rights and permissions
About this article
Cite this article
Macpherson, M., Hamrén, B., Braamskamp, M.J.A.M. et al. Population pharmacokinetics of rosuvastatin in pediatric patients with heterozygous familial hypercholesterolemia. Eur J Clin Pharmacol 72, 19–27 (2016). https://doi.org/10.1007/s00228-015-1946-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1946-4