Abstract
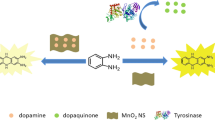
Tyrosinase (TYR), as an important biological enzyme, has been widely used in synthetic biology, medical hairdressing, environmental detection, biological sensors, and other fields. In clinical practice, tyrosinase activity is an important indicator for detecting melanoma. Therefore, the detection of tyrosinase activity is of great importance. Based on the polyphenol oxidase activity of tyrosinase, a simple and rapid detection method was proposed based on the adjustable light scattering properties of cobalt hydroxyl oxide nanoflakes (CoOOH NFs). It was found that the amount and size of CoOOH NFs decreased due to the redox reaction mediated by catechol (CC), resulting in a lower light scattering signal of CoOOH NFs. However, in the presence of tyrosinase, catechol was oxidized to a quinone structure, resulting in the reduced decomposition of CoOOH NFs and recovered light scattering signal, which was developed for the quantitative detection of tyrosinase activity. It was found that in the range of 10–400 U/L, the light scattering intensity was correlated linearly with tyrosinase activity, and the limit of detection was 6.71 U/L (3σ/k). To verify the feasibility of the proposed method in clinical samples, the spiked recovery experiments were carried out with human serum samples, which showed recovery rates between 93.0% and 104.6%, suggesting the high accuracy. The proposed assay provides a simple and rapid method for detection of a natural enzyme based on the adjustable light scattering properties of CoOOH nanoflakes, which lays the foundation for the development of various enzyme sensing applications in the future.
Graphical abstract










Similar content being viewed by others
Abbreviations
- UCNPs:
-
Upconversion nanoparticles
- NPs:
-
Nanoparticles
- APBA-QDs:
-
3-Aminophenyl boric acid-functionalized quantum dots
- PDA:
-
Polydopamine
References
Noh H, Lee SJ, Jo HJ, Choi HW, Hong S, Kong KH. Histidine residues at the copper-binding site in human tyrosinase are essential for its catalytic activities. J Enzyme Inhib Med Chem. 2020;35:726–32.
Rolff M, Schottenheim J, Decker H, Tuczek F. Copper-O2 reactivity of tyrosinase models towards exteRNAl monophenolic substrates: molecular mechanism and comparison with the enzyme. Chem Soc Rev. 2011;40:4077–98.
Loizzo MR, Tundis R, Menichini F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: an update. Compr Rev Food Sci Food Saf. 2012;11:378–98.
Li XQ, Li SQ, Liang XP, McClements DJ, Liu XB, Liu FG. Applications of oxidases in modification of food molecules and colloidal systems: laccase, peroxidase and tyrosinase. Trends Food Sci Tech. 2020;103:78–93.
Yu FF, Lu YS, Zhong ZM, Qu BL, Wang MF, Yu XY, Chen JY. Mitf involved in innate immunity by activating tyrosinase-mediated melanin synthesis in pteria penguin. Front Immunol. 2021;12: 626493.
Jantakee K, Tragoolpua Y. Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biol Res. 2015;48:4.
Odonbayar B, Murata T, Batkhuu J, Yasunaga K, Goto R, Sasakit K. Antioxidant flavonols and phenolic compounds from atraphaxis frutescens and their inhibitory activities against insect phenoloxidase and mushroom tyrosinase. J Nat Prod. 2016;79:3065–71.
Pu YY, Zhou BG, **ang HJ, Wu WC, Yin HH, Yue WW, Yin YF, Li HY, Chen Y, Xu HX. Tyrosinase-activated prodrug nanomedicine as oxidative stress amplifier for melanoma-specific treatment. Biomaterials. 2020;259: 120329.
Niu C, Aisa HA. Upregulation of melanogenesis and tyrosinase activity: potential agents for vitiligo. Molecules. 2017;22:1303.
Arrowitz C, Schoelermann AM, Mann T, Jiang LI, Weber T, Kolbe L. Effective tyrosinase inhibition by thiamidol results in significant improvement of mild to moderate melasma. J Invest Dermatol. 2019;139:1691–8.
Yang S, Jiang JX, Zhou AX, Zhou YB, Ye WL, Cao DS, Yang RH. Substrate-photocaged enzymatic fluorogenic probe enabling sequential activation for light-controllable monitoring of intracellular tyrosinase activity. Anal Chem. 2020;92:7194–9.
Wu XF, Li LH, Shi W, Gong QY, Ma HM. Near-infrared fluorescent probe with new recognition moiety for specific detection of tyrosinase activity: design, synthesis, and application in living cells and zebrafish. Angew Chem-In Ed. 2016;55:14728–32.
Li SQ, Liu D, Wu BY, Sun HP, Liu XY, Zhang HX, Ding NN, Wu L. One-pot synthesis of a peroxidase-like nanozyme and its application in visual assay for tyrosinase activity. Talanta. 2022;239: 123088.
Li J, Wei YY, Liu XP, Xu ZR. Plasmonic photothermal biosensor for visual detection of tyrosinase and dopamine based on manganese dioxide nanosheets-mediated etching of gold nanorods. Sensor Actuat B- Chem. 2022;353: 131139.
Shelef O, Sedgwick AC, Pozzi S, Green O, Satchi-Fainaro R, Shabat D, Sessler JL. Turn on chemiluminescence-based probes for monitoring tyrosinase activity in conjunction with biological thiols. Chem Commun. 2021;57:11386–9.
Erkmen C, Demir Y, Kurbanoglu S, Uslu B. Multi-purpose electrochemical tyrosinase nanobiosensor based on poly(3,4 ethylenedioxythiophene) nanoparticles decorated graphene quantum dots: applications to hormone drugs analyses and inhibition studies. Sensor Actuat B-Chem. 2021;343: 130164.
Zhuang XM, Hu YJ, Wang JJ, Hu JY, Wang Q, Yu XX. A colorimetric and SERS dual-readout sensor for sensitive detection of tyrosinase activity based on 4-mercaptophenyl boronic acid modified AuNPs. Anal Chim Acta. 2021;1188: 339172.
Yildiz HB, Freeman R, Gill R, Willner I. Electrochemical, photoelectrochemical, and piezoelectric analysis of tyrosinase activity by functionalized nanoparticles. Anal Chem. 2008;80:2811–6.
Munyemana JC, Chen J, Tang H, Han YX, Wang JJ, Qiu HD. Discriminative detection of dopamine and tyrosinase based on polydopamine dots triggered by Fenton-like activity of Mn3O4 nanoparticles. ACS Appl Nano Mater. 2021;4:2820–7.
Lemineur JF, Stockmann TJ, Medard J, Smadja C, Combellas C, Kanoufi F. Optical nanoimpacts of dielectric and metallic nanoparticles on gold surface by reflectance microscopy: adsorption or bouncing? J Anal Test. 2019;3:175–88.
Mao ZY, Zhu LN, Gao J, Liu JJ, Wei YH, Li XY, Yin BC, Wang J. A CoOOH nanoflake-based light scattering probe for the simple and selective detection of uric acid in human serum. Anal Methods. 2018;10:4951–7.
Wei YH, Li XY, Gao J, Liu JJ, Yuan D, Yin BC, Wang J. Size-dependent modulation of CoOOH nanoflakes light scattering for rapid and selective detection of tetracycline in milk. J Anal Test. 2018;2:332–41.
Zhu LN, Cheng R, Kang KW, Chen MY, Zhan T, Wang J. Size-dependent light scattering of CoOOH nanoflakes for convenient and sensitive detection of alkaline phosphatase in human serum. Luminescence. 2021;36:1317–26.
Li ZM, Zhong XL, Wen SH, Zhang L, Liang RP, Qiu JD. Colorimetric detection of methyltransferase activity based on the enhancement of CoOOH nanozyme activity by ssdna. Sensor Actuat B- Chem. 2019;281:1073–9.
Wu ZH, Nan DY, Yang H, Pan S, Liu H, Hu XL. A ratiometric fluorescence-scattered light strategy based on MoS2 quantum dots/CoOOH nanoflakes system for ascorbic acid detection. Anal Chim Acta. 2019;1091:59–68.
Prexler SM, Frassek M, Moerschbacher BM, Dirks-Hofmeister ME. Catechol oxidase versus tyrosinase classification revisited by site-directed mutagenesis studies. Angew Chem-In Ed. 2019;58:8757–61.
Craig DB, Hiebert Z. Analysis of complexes of metabolites with europium tetracycline using capillary electrophoresis coupled with laser-induced luminescence detection. Biometals. 2017;30:449–58.
Chen Z, Wang X, Keßler S, Fan Q, Huang M, Cölfen H. Synthesis of hierarchical transition metal oxyhydroxides in aqueous solution at ambient temperature and their application as OER electrocatalysts. J Energy Chem. 2022;71:89–97.
Li L, Wang C, Liu K, Wang Y, Liu K, Lin Y. Hexagonal cobalt oxyhydroxide–carbon dots hybridized surface: high sensitive fluorescence turn-on probe for monitoring of ascorbic acid in rat brain following brain ischemia. Anal Chem. 2015;87:3404–11.
Zou BQ, Zhang HZ, Fu Z, Zhan T, Wang J. Size-modulated optical property of gold nanorods for sensitive and colorimetric detection of thiourea in fruit juice. Talanta. 2021;225: 121965.
Liu JJ, Yan HH, Zhang Q, Gao PF, Li CM, Liang GL, Huang CZ, Wang J. High-resolution vertical polarization excited dark-field microscopic imaging of anisotropic gold nanorods for the sensitive detection and spatial imaging of intracellular microRNA-21. Anal Chem. 2020;92:13118–25.
Liu JJ, Wen S, Yan HH, Cheng R, Zhu F, Gao PF, Zou HY, Huang CZ, Wang J. The accurate imaging of collective gold nanorods with a polarization-dependent dark-field light scattering microscope. Anal Chem. 2023;95:1169–75.
**a J, Yang Y, Jiang X, Shabbir M, Luo X. Cobalt oxyhydroxide nanoflakes/cellulose composite membranes with enhanced detection of ascorbic acid. ACS Appl Polym Mater. 2022;4:469–78.
Munjal N, Sawhney SK. Stability and properties of mushroom tyrosinase entrapped in alginate, poyacrylamide and gelatin gels. Enzyme Microb Technol. 2002;30:613–9.
Liu G, Zhao J, Lu S, Wang S, Sun J, Yang X. Polymethyldopa nanoparticles-based fluorescent sensor for detection of tyrosinase activity. ACS Sens. 2018;3:1855–62.
Cen Y, Tang J, Kong X-J, Wu S, Yuan J, Yu R-Q, Chu X. A cobalt oxyhydroxide-modified upconversion nanosystem for sensitive fluorescence sensing of ascorbic acid in human plasma. Nanoscale. 2015;7:13951–7.
Wang M, **e JL, Li J, Fan YY, Deng X, Duan HL, Zhang ZQ. 3-Aminophenyl boronic acid functionalized quantum-dot-based ratiometric fluorescence sensor for the highly sensitive detection of tyrosinase activity. ACS Sens. 2020;5:1634–40.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22174115) and the Graduate Education and Teaching Reform Research Project of Chongqing (No. yjg223038).
This work contains human blood samples from volunteers at Southwest University, which operated according to the ethical standards of the Ethics Committee of Southwest University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry 2023 with guest editors Zhi-Yuan Gu, Beatriz Jurado-Sánchez, Thomas H. Linz, Leandro Wang Hantao, Nongnoot Wongkaew, and Peng Wu.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M.X., Kang, K.W., Huang, M. et al. Simple and rapid detection of tyrosinase activity with the adjustable light scattering properties of CoOOH nanoflakes. Anal Bioanal Chem 415, 4569–4578 (2023). https://doi.org/10.1007/s00216-023-04710-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04710-x