Abstract
Recent state-of-the-art methods developed for the analysis of polar xenobiotics from different types of biological matrices usually employ liquid chromatography with mass spectrometry. However, there are limitations when a small amount of sample mass is available. For example, individual benthic invertebrates or fish tissue samples often weigh less than 100 mg (e.g., brain, liver) but are necessary to understand environmental fate and bioaccumulation dynamics. We developed ultra-fast methods based on a direct sample introduction technique. This included coupling laser diode thermal desorption with atmospheric pressure chemical ionization mass spectrometry (LDTD-APCI-MS). We then quantitated a common selective serotonin reuptake inhibitor (citalopram) in brain tissues of individual juvenile fish after in vivo exposure to environmentally relevant concentration. Two mass spectrometric methods based on low (LDTD-APCI-triple quadrupole (QqQ)-MS/MS) and high (LDTD-APCI-high-resolution product scan (HRPS)) resolutions were developed and evaluated. Individual instrument conditions were optimized to achieve an accurate and robust analytical method with minimum sample preparation requirements. We achieved very good recovery (97–108%) across the range of 1–100 ng g−1 for LDTD-APCI-HRPS. LDTD-APCI-QqQ-MS/MS showed poorer performance due to interferences from the matrix at the lowest concentration level. LDTD-APCI ionization was successfully validated for analysis of non-filtered sample extracts. Evaluation of final methods was performed for a set of real fish brain samples, including comparison of LDTD-APCI-HRPS with a previously validated LC-heated electrospray ionization-HRPS method. This new LDTD-APCI-HRPS method avoids the chromatographic step and provides important benefits such as analysis of limited sample masses, lower total sample volume (typically μL), and reduction in analysis time per sample run to a few seconds.

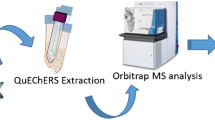
Graphical abstract







Similar content being viewed by others
References
Carter LJ, Harris E, Williams M, Ryan JJ, Kookana RS, Boxall ABA. Fate and uptake of pharmaceuticals in soil–plant systems. J Agric Food Chem. 2014;62:816–25. https://doi.org/10.1021/jf404282y.
Agunbiade FO, Moodley B. Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ Toxicol Chem. 2016;35:36–46. https://doi.org/10.1002/etc.3144.
Gualano MR, Bert F, Mannocci A, La Torre G, Zeppegno P, Siliquini R. Consumption of antidepressants in Italy: recent trends and their significance for public health. Psychiatr Serv. 2014;65:1226–31. https://doi.org/10.1176/appi.ps.201300510.
Ilyas S, Moncrieff J. Trends in prescriptions and costs of drugs for mental disorders in England, 1998-2010. Br J Psychiatry. 2012;200:393–8. https://doi.org/10.1192/bjp.bp.111.104257.
Státní ústav pro kontrolu léčiv. http://www.sukl.cz/. Accessed 22 Oct 2019.
Mackulak T, Mosny M, Skubak J, Grabic R, Birosova L. Fate of psychoactive compounds in wastewater treatment plant and the possibility of their degradation using aquatic plants. Environ Toxicol Pharmacol. 2015;39:969–73. https://doi.org/10.1016/j.etap.2015.02.018.
Golovko O, Kumar V, Fedorova G, Randak T, Grabic R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere. 2014;111:418–26. https://doi.org/10.1016/J.CHEMOSPHERE.2014.03.132.
Gurke R, Rößler M, Marx C, Diamond S, Schubert S, Oertel R, et al. Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci Total Environ. 2015;532:762–70. https://doi.org/10.1016/j.scitotenv.2015.06.067.
Bradley PM, Barber LB, Clark JM, Duris JW, Foreman WT, Furlong ET, et al. Pre/post-closure assessment of groundwater pharmaceutical fate in a wastewater-facility-impacted stream reach. Sci Total Environ. 2016;568:916–25. https://doi.org/10.1016/j.scitotenv.2016.06.104.
González Alonso S, Catalá M, Maroto RR, Gil JLR, de Miguel ÁG, Valcárcel Y. Pollution by psychoactive pharmaceuticals in the rivers of Madrid metropolitan area (Spain). Environ Int. 2010;36:195–201. https://doi.org/10.1016/j.envint.2009.11.004.
Mackuľak T, Bodík I, Hasan J, Grabic R, Golovko O, Vojs-Staňová A, et al. Dominant psychoactive drugs in the Central European region: a wastewater study. Forensic Sci Int. 2016;267:42–51. https://doi.org/10.1016/J.FORSCIINT.2016.08.016.
Mole RA, Brooks BW. Global scanning of selective serotonin reuptake inhibitors: occurrence, wastewater treatment and hazards in aquatic systems. Environ Pollut. 2019:1019–31.
Grabicova K, Lindberg RH, Östman M, Grabic R, Randak T, Joakim Larsson DG, et al. Tissue-specific bioconcentration of antidepressants in fish exposed to effluent from a municipal sewage treatment plant. Sci Total Environ. 2014;488:46–50. https://doi.org/10.1016/j.scitotenv.2014.04.052.
Grabicova K, Grabic R, Fedorova G, Fick J, Cerveny D, Kolarova J, et al. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream. Water Res. 2017;124:654–62. https://doi.org/10.1016/j.watres.2017.08.018.
Grabicova K, Grabic R, Blaha M, Kumar V, Cerveny D, Fedorova G, et al. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015;72:145–53. https://doi.org/10.1016/j.watres.2014.09.018.
Cunha DL, Mendes MP, Marques M. Environmental risk assessment of psychoactive drugs in the aquatic environment. Environ Sci Pollut Res. 2019;26:78–90.
Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243:55–60. https://doi.org/10.1016/J.FORSCIINT.2014.04.017.
Marchei E, Escuder D, Pallas CR, Garcia-Algar O, Gómez A, Friguls B, et al. Simultaneous analysis of frequently used licit and illicit psychoactive drugs in breast milk by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2011;55:309–16. https://doi.org/10.1016/J.JPBA.2011.01.028.
Mallett DN, Ramírez-Molina C. The use of partially porous particle columns for the routine, generic analysis of biological samples for pharmacokinetic studies in drug discovery by reversed-phase ultra-high performance liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2009;49:100–7. https://doi.org/10.1016/J.JPBA.2008.09.041.
Ali AM, Rønning HT, Sydnes LK, Alarif WM, Kallenborn R, Al-Lihaibi SS. Detection of PPCPs in marine organisms from contaminated coastal waters of the Saudi Red Sea. Sci Total Environ. 2018;621:654–62. https://doi.org/10.1016/j.scitotenv.2017.11.298.
Poliakoff M, Fitzpatrick JM, Farren TR, Anastas PT (2002) Green chemistry: science and politics of change. Science (80-. )
Erythropel HC, Zimmerman JB, De Winter TM, Petitjean L, Melnikov F, Lam CH, et al. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018;20:1929–61.
Roy-Lachapelle A, Solliec M, Sinotte M, Deblois C, Sauvé S. Total analysis of microcystins in fish tissue using laser thermal desorption–atmospheric pressure chemical ionization–high-resolution mass spectrometry (LDTD-APCI-HRMS). J Agric Food Chem. 2015;63:7440–9. https://doi.org/10.1021/acs.jafc.5b02318.
Munoz G, Vo Duy S, Budzinski H, Labadie P, Liu J, Sauvé S. Quantitative analysis of poly- and perfluoroalkyl compounds in water matrices using high resolution mass spectrometry: optimization for a laser diode thermal desorption method. Anal Chim Acta. 2015;881:98–106. https://doi.org/10.1016/j.aca.2015.04.015.
Lonappan L, Pulicharla R, Rouissi T, Brar SK, Verma M, Surampalli RY, et al. Diclofenac in municipal wastewater treatment plant: quantification using laser diode thermal desorption—atmospheric pressure chemical ionization—tandem mass spectrometry approach in comparison with an established liquid chromatography-electrospray ionization–tandem mass spectrometry method. J Chromatogr A. 2016;1433:106–13. https://doi.org/10.1016/J.CHROMA.2016.01.030
Mohapatra DP, Brar SK, Tyagi RD, Picard P, Surampalli RY. Carbamazepine in municipal wastewater and wastewater sludge: ultrafast quantification by laser diode thermal desorption-atmospheric pressure chemical ionization coupled with tandem mass spectrometry. Talanta. 2012;99:247–55. https://doi.org/10.1016/J.TALANTA.2012.05.047.
Swales JG, Gallagher R, Peter RM. Determination of metformin in mouse, rat, dog and human plasma samples by laser diode thermal desorption/atmospheric pressure chemical ionization tandem mass spectrometry. J Pharm Biomed Anal. 2010;53:740–4. https://doi.org/10.1016/J.JPBA.2010.04.033.
Heudi O, Barteau S, Picard P, Tremblay P, Picard F, Kretz O. Laser diode thermal desorption–positive mode atmospheric pressure chemical ionization tandem mass spectrometry for the ultra-fast quantification of a pharmaceutical compound in human plasma. J Pharm Biomed Anal. 2011;54:1088–95. https://doi.org/10.1016/J.JPBA.2010.11.025.
Borik A, Vojs Stanova A, Kodesova R, Brooks BW, Grabicova K, Novakova P, et al. Ultrafast laser diode thermal desorption method for analysis of representative pharmaceuticals in soil leachate samples. Talanta. 2020;208:120382. https://doi.org/10.1016/j.talanta.2019.120382.
Grabicova K, Staňová AV, Koba O, Borik A, Randak T, Grabic R. Development of a robust extraction procedure for the HPLC-ESI-HRPS determination of multi-residual pharmaceuticals in biota samples. Anal Chim Acta. 2018. https://doi.org/10.1016/j.aca.2018.04.011.
Solliec M, Massé D, Sauvé S. Analysis of trimethoprim, lincomycin, sulfadoxin and tylosin in swine manure using laser diode thermal desorption-atmospheric pressure chemical ionization-tandem mass spectrometry. Talanta. 2014;128:23–30. https://doi.org/10.1016/J.TALANTA.2014.04.023.
Kruve A, Rebane R, Kipper K, Oldekop M-L, Evard H, Herodes K, et al. Tutorial review on validation of liquid chromatography–mass spectrometry methods: part II. Anal Chim Acta. 2015;870:8–28. https://doi.org/10.1016/J.ACA.2015.02.016.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30. https://doi.org/10.1021/ac020361s.
Passing H, Bablok W. Application of statistical procedures in analytical instrument testing. J Automat Chem. 1985;7:74–9.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England). 1986;1:307–10.
Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics. 2012;11:1475–88. https://doi.org/10.1074/mcp.O112.020131.
Giavarina D. Understanding Bland Altman analysis. Biochem Medica. 2015;25:141–51. https://doi.org/10.11613/BM.2015.015.
Lidija B-Z. Comparison of methods: Passing and Bablok regression. Biochem Medica. 2011;21:49–52.
Roy-Lachapelle A, Solliec M, Sinotte M, Deblois C, Sauvé S. High resolution/accurate mass (HRMS) detection of anatoxin-a in lake water using LDTD–APCI coupled to a Q-Exactive mass spectrometer. Talanta. 2015;132:836–44. https://doi.org/10.1016/J.TALANTA.2014.10.021.
Allen KR. Interference by venlafaxine ingestion in the detection of tramadol by liquid chromatography linked to tandem mass spectrometry for the screening of illicit drugs in human urine. Clin Toxicol. 2006;44:147–53. https://doi.org/10.1080/15563650500514434.
Viant MR, Davis JE, Duffy C, Engel J, Stenton C, Sebire M, et al. Application of passive sampling to characterise the fish exometabolome. Metabolites. 2017;7. https://doi.org/10.3390/metabo7010008.
Young T, Walker SP, Alfaro AC, Fletcher LM, Murray JS, Lulijwa R, et al. Impact of acute handling stress, anaesthesia, and euthanasia on fish plasma biochemistry: implications for veterinary screening and metabolomic sampling. Fish Physiol Biochem. 2019;45:1485–94. https://doi.org/10.1007/s10695-019-00669-8.
Sogin EM, Puskás E, Dubilier N, Liebeke M. Marine metabolomics: a method for nontargeted measurement of metabolites in seawater by gas chromatography–mass spectrometry. mSystems. 2019:4. https://doi.org/10.1128/msystems.00638-19.
Clendinen CS, Monge ME, Fernández FM. Ambient mass spectrometry in metabolomics. Analyst. 2017;142:3101–17.
Funding
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic projects “CENAKVA” (LM2018099) and “CENAKVA Center Development (No. CZ.1.05/2.1.00/19.0380)” and by the Czech Science Foundation (No. 18-15802S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
This experiment was performed in accordance with the EU-harmonized Animal Welfare Act of the Czech Republic. The research facility is authorized under (No. 53100/2013-MZE-17214) the framework of the law against Animal Cruelty of the Czech Republic (No. 246/1992), with the ethical approval committee number MSMT-6744/2018-4.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 420 kb)
Rights and permissions
About this article
Cite this article
Borik, A., Staňová, A.V., Brooks, B.W. et al. Determination of citalopram in fish brain tissue: benefits of coupling laser diode thermal desorption with low- and high-resolution mass spectrometry. Anal Bioanal Chem 412, 4353–4361 (2020). https://doi.org/10.1007/s00216-020-02672-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02672-y