Abstract
A candidate reference measurement procedure (RMP) based on isotope dilution coupled with liquid chromatography–tandem mass spectrometry (ID–LC–MS/MS) was developed and validated for the quantification of 17α-hydroxyprogesterone (17-OHP) in human plasma. 17-OHP spiked with a deuterium-labeled internal standard was extracted from plasma by liquid–liquid extraction with 1 mL n-hexane/ethyl acetate (3:2, v/v). Reversed-phase chromatography and positive electrospray ionization were used in the ID–LC–MS/MS. Gradient elution coupled with use of a C18-packed ultrahigh-performance liquid chromatography column allowed complete baseline resolution of 17-OHP from its structural analogue desoxycorticosterone in 6 min. To determine the 17-OHP level in human plasma, a bracketing calibration method was used to give higher accuracy and precision. The limit of detection and the lower limit of the measuring interval for the candidate RMP were 2.1 pg/mL (6.4 pmol/L) and 4.6 pg/mL (13.9 pmol/L), respectively. Extraction recovery was determined to be (96.08 ± 3.03)% (n = 3). Imprecision (intra-assay and interassay) was 4.03% or less at 0.83, 15.19, 64.22, and 313.46 ng/mL (2.51, 45.97, 194.34, and 948.56 nmol/L, respectively). Recoveries ranged from 98.05% to 102.24%. When comparing our RMP results with those obtained with an established RMP via International Federation of Clinical Chemistry and Laboratory Medicine external quality assessment scheme for reference laboratories in laboratory medicine (RELA) samples, we found that the biases ranged from -1.99% to 3.08% against the targets. No interference was observed, and the linear response ranged from 0.47 to 958.63 ng/mL (1.42 to 2900.90 nmol/L). Moreover, the candidate RMP was used to measure the concentration of 17-OHP in human plasma and was compared with an immunoassay using 40 plasma samples. The performance of the method meets the needs of an RMP (total coefficient of variation of 5% or less and bias of 3.08% or less). This method can be used for reference material value assignment of 17-OHP in human plasma matrix. It could also serve as an accurate reference baseline for routine methods to increase the accuracy and precision of certain clinical laboratory measurements.

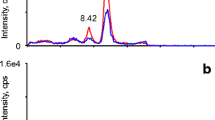
Selected ion chromatograms obtained by liquid chromatography–tandem mass spectrometry with a C18 column for 17α-hydroxyprogesterone (17-OHP) from a plasma sample





Similar content being viewed by others
Abbreviations
- 17-OHP:
-
17α-Hydroxyprogesterone
- CAH:
-
Congenital adrenal hyperplasia
- CLSI:
-
Clinical and Laboratory Standards Institute
- cRMP:
-
Candidate reference measurement procedure
- CV:
-
Coefficient of variation
- IFCC:
-
International Federation of Clinical Chemistry and Laboratory Medicine
- ISO:
-
International Organization for Standardization
- JCTLM:
-
Joint Committee for Traceability in Laboratory Medicine
- LC:
-
Liquid chromatography
- LLMI:
-
Lower limit of the measuring interval
- LoD:
-
Limit of detection
- MS:
-
Mass spectrometry
- MS/MS:
-
Tandem mass spectrometry
- RELA:
-
International Federation of Clinical Chemistry and Laboratory Medicine external quality assessment scheme for reference laboratories in laboratory medicine
- RfB:
-
Referenzinstitut für Bioanalytik
- RMP:
-
Reference measurement procedure
References
Reinehr T, Rothermel J, Wegener-Panzer A, Hartmann MF, Wudy SA, Holterhus PM. Vanishing 17-hydroxyprogesterone concentrations in 21-hydroxylase deficiency. Horm Res Paediatr. 2018;90(2):138–44.
De Jesus VR, Simms DA, Schiffer J, Kennedy M, Mei JV, Hannon WH. Pilot proficiency testing study for second tier congenital adrenal hyperplasia newborn screening. Clin Chim Acta. 2010;411(21-22):1684–7.
Grandone A, Marzuillo P, Luongo C, Toraldo R, Mariani M, Miraglia DGE, et al. Basal levels of 17-hydroxyprogesterone can distinguish children with isolated precocious pubarche. Pediatr Res. 2018;84(4):533–6.
Witchel SF. Newborn screening for congenital adrenal hyperplasia: beyond 17-hydroxyprogesterone concentrations. J Pediatr. 2019;95(3):257–9.
Setji TL, Brown AJ. Polycystic ovary syndrome: update on diagnosis and treatment. Am J Med. 2014;127(10):912–9.
Taieb J, Benattar C, Birr AS, Lindenbaum A. Limitations of steroid determination by direct immunoassay. Clin Chem. 2002;48(3):583–5.
Speiser PW. Improving neonatal screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89(8):3685–6.
Fingerhut R. False positive rate in newborn screening for congenital adrenal hyperplasia (CAH)-ether extraction reveals two distinct reasons for elevated 17α-hydroxyprogesterone (17-OHP) values. Steroids. 2009;74(8):662–5.
Kosel S, Burggraf S, Fingerhut R, Dorr HG, Roscher AA, Olgemoller B. Rapid second-tier molecular genetic analysis for congenital adrenal hyperplasia attributable to steroid 21-hydroxylase deficiency. Clin Chem. 2005;51(2):298–304.
Botelho JC, Ribera A, Cooper HC, Vesper HW. Evaluation of an isotope dilution HPLC tandem mass spectrometry candidate reference measurement procedure for total 17-β estradiol in human serum. Anal Chem. 2016;88(22):11123–9.
Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441–8.
Huang X, Zhang Q, Zheng S, Wang J, Han L, Lin H, et al. Measurement of human serum unconjugated estriol without derivatization using liquid chromatography-tandem mass spectrometry candidate reference method and compared with two immunoassays. Anal Bioanal Chem. 2018;410(24):6257–67.
International Organization for Standardization. In vitro diagnostic medical devices — requirements for establishing metrological traceability of values assigned to calibrators, trueness control materials and human samples. ISO 17511. Geneva: International Organization for Standardization; 2019.
Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Owen WE, Bunker AM, et al. Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin Chem. 2006;52(8):1559–67.
Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem. 2004;50(3):621–5.
Etter ML, Eichhorst J, Lehotay DC. Clinical determination of 17-hydroxyprogesterone in serum by LC-MS/MS: comparison to Coat-A-Count RIA method. J Chromatogr B Anal Technol Biomed Life Sci. 2006;840(1):69–74.
Magnisali P, Dracopoulou M, Mataragas M, Dacou-Voutetakis A, Moutsatsou P. Routine method for the simultaneous quantification of 17α-hydroxyprogesterone, testosterone, dehydroepiandrosterone, androstenedione, cortisol, and pregnenolone in human serum of neonates using gas chromatography-mass spectrometry. J Chromatogr A. 2008;1206(2):166–77.
Han L, Tavakoli NP, Morrissey M, Spink DC, Cao ZT. Liquid chromatography-tandem mass spectrometry analysis of 17-hydroxyprogesterone in dried blood spots revealed matrix effect on immunoassay. Anal Bioanal Chem. 2019;411(2):395–402.
International Organization for Standardization. In vitro diagnostic medical devices: measurement of quantities in samples of biological origin–requirements for content and presentation of reference measurement procedures. ISO 15193. Geneva: International Organization for Standardization; 2009.
Thienpont LM. Quality specifications for reference methods. Scand J Clin Lab Invest. 1999;59(7):535–8.
Wudy SA, Hartmann M, Svoboda M. Determination of 17-hydroxyprogesterone in plasma by stable isotope dilution/benchtop liquid chromatography-tandem mass spectrometry. Horm Res. 2000;53(2):68–71.
Joint Committee for Traceability of Laboratory Medicine. Database of higher order reference materials; measurement methods/procedures and services. http://www.bipm.org/jctlm/. Accessed Jan 2019.
Clinical and Laboratory Standards Institute. Liquid chromatography-mass spectrometry methods; approved guideline. Document C62-A. Wayne: Clinical and Laboratory Standards Institute; 2014.
Clinical and Laboratory Standards Institute. Preliminary evaluation of quantitative clinical laboratory methods; approved guideline, 3rd edition. Document EP10-A3. Wayne: Clinical and Laboratory Standards Institute; 2014.
Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. Document EP06. Wayne: Clinical and Laboratory Standards Institute; 2003.
Zhang Q, Han L, Wang J, Lin H, Ke P, Zhuang J, et al. Simultaneous quantitation of endogenous estrone, 17β-estradiol, and estriol in human serum by isotope-dilution liquid chromatography–tandem mass spectrometry for clinical laboratory applications. Anal Bioanal Chem. 2017;409(10):2627–38.
Stockl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta. 2009;408(1-2):8–13.
Fraser CG, Kallner A, Kenny D, Petersen PH. Introduction: strategies to set global quality specifications in laboratory medicine. Scand J Clin Lab Invest. 1999;59(7):477–8.
Stockl D, Reinauer H. Development of criteria for the evaluation of reference method values. Scand J Clin Lab Investig Suppl. 1993;212:16–8.
National Center for Clinical Laboratories (NCCL). External Quality Assessment Programs in Laboratory Medicine; 2019 Page 57. https://www.nccl.org.cn/showEqaPtDetail?id=23
Machacek DA., Singh RJ. Development and validation of serum 17 alpha-hydroxy progesterone LC-MS/MS assay for screening and monitoring of congenital adrenal hyperplasia, presented at American Society of Mass Spectrometry meeting, 2001, Chicago.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2017YFF0205401), the National Natural Science Foundation of China (81572088), the Natural Science Foundation of Guangdong Province (2018A0303130124), the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (YN2016QJ15 and YN2019QL01), and the Guangzhou Science and Technology Project (201704020213).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving humans and/or animals
The plasma samples were taken from pregnant women and this study is approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. The number of this review is B2016-159-01.
Informed consent
The Ethics Committee approved the exemption of informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 485 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, L., Lin, H. et al. Evaluation of a bracketing calibration-based isotope dilution liquid chromatography–tandem mass spectrometry candidate reference measurement procedure for 17α-hydroxyprogesterone in human plasma. Anal Bioanal Chem 411, 7095–7104 (2019). https://doi.org/10.1007/s00216-019-02086-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02086-5