Abstract
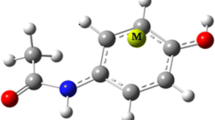
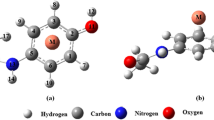
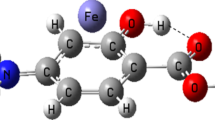
Density functional theory calculations are performed to determine the effect of cation-π and intramolecular hydrogen bond (IMHB) interactions on each other in the formed complexes between transition metal cations (Mn+, Fe2+, Co+, Ni2+, Cu+, Zn2+) with mesalazine drug. The strength of these interactions is evaluated by energetic, geometric, spectroscopic and topological parameters to explore the mutual effects between them. Atomic charge distribution and characterization of bonds in the studied systems are investigated by natural bond orbital and atoms in molecules analyses, respectively. Our findings show that the presence of IMHB increases the energies of cation–π interaction for the divalent complexes and Co+ complex, while for the other monovalent complexes the reverse process is observed. The results also display that, in most cases, the coexistence of IMHB and cation–π interactions decreases the IMHB strength.






Similar content being viewed by others
References
Iacucci M, de Silva Sh, Ghosh S (2010) Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol 24:127–133
Azad Khan AK, Piris J, Truelove SC (1977) An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 2:892–895
Hanauer SB (1990) Topical and Oral Aminosalicylates. In: Peppercorn M (ed) The Management of Inflammatory Bowel Disease: New Medical and Surgical Approaches. Marcel Dekker, Philadelphia
Miles AM, Grisham MB (1995) Antioxidant properties of 5-aminosalicylic acid: potential mechanism for its protective effect in ulcerative colitis. Adv Exp Med Biol 371B:1317–1321
Muller-Dethlefs K, Hobza P (2000) Noncovalent interactions: a challenge for experiment and theory. Chem Rev 100:143–167
Strekowski L, Wilson B (2007) Noncovalent interactions with DNA: an overview. Mutat Res 623:3–13
Esrafili MD, Behzadi H, Hadipour NL (2007) Influence of N–H…O and O–H…O hydrogen bonds on the 17O, 15N and 13C chemical shielding tensors in crystalline acetaminophen: a density functional theory study. Biophys Chem 128:38–45
Grimme S (2008) Do special noncovalent π–π stacking interactions really exist? Angew Chem Int Ed 47:3430–3434
Esrafili MD, Behzadi H, Beheshtian J, Hadipour NL (2008) Theoretical 14N nuclear quadrupole resonance parameters for sulfa drugs: Sulfamerazine and sulfathiazole. J Mol Graph Model 27:326–331
Jeffrey GA (1997) An Introduction to Hydrogen Bonding. Oxford University Press, New York
Desiraju GR (2002) Hydrogen bridges in crystal engineering: interactions without borders. Acc Chem Res 35:565–573
Pauling L (1939) The Nature of the Chemical Bond. Cornell University Press, Ithaca, NY
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76
Israelachvili JN (1992) Intermolecular and surface forces. Academic Press, London
Reddy AS, Sastry GN (2005) Cation [M = H+, Li+, Na+, K+, Ca2+, Mg2+, NH4+, and NMe4+] interactions with the aromatic motifs of naturally occurring amino acids: a theoretical study. J Phys Chem A 109:8893–8903
Gokel GW, De Wall SL, Meadows ES (2000) Experimental evidence for alkali metal cation−π interactions. Eur J Org Chem 2000:2967–2978
Gokel GW, Barbour LJ, De Wall SL, Meadows ES (2001) Macrocyclic polyethers as probes to assess and understand alkali metal cation-π interactions. Coord Chem Rev 222:127–154
Ma JC, Dougherty DA (1997) The cation–π interaction. Chem Rev 97:1303–1324
Subha Mahadevi A, Narahari Sastry G (2013) Cation−π interaction: its role and relevance in chemistry, biology, and material science. Chem Rev 113:2100–2138
Shinkai S, Ikeda M, Sugasaki A, Takeuchi M (2001) Positive allosteric systems designed on dynamic supramolecular scaffolds: toward switching and amplification of guest affinity and selectivity. Acc Chem Res 34:494–503
Meyer EA, Castellano RK, Diederich F (2003) Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed 42:1210–1250
Mulder A, Huskens J, Reinhoudt DN (2004) Multivalency in supramolecular chemistry and nanofabrication. Org Biomol Chem 2:3409–3424
Estarellas C, Escudero D, Frontera A, Quiñonero D, Deyá PM (2009) Theoretical ab initio study of the interplay between hydrogen bonding, cation–π and π–π interactions. Theor Chem Acc 122:325–332
Estarellas C, Frontera A, Quiñonero D, Deyá PM (2009) Interplay between cation–π and hydrogen bonding interactions: are non-additivity effects additive? Chem Phys Lett 479:316–320
Escudero D, Frontera A, Quiñonero D, Deyá PM (2008) Interplay between cation-π and hydrogen bonding interactions. Chem Phys Lett 456:257–261
Nowroozi A, Ebrahimi A, Rezvani Rad O (2018) Mutual effects of the cation-π, anion-π and intramolecular hydrogen bond in the various complexes of 1,3,5-triamino-2,4,6-trinitrobenzene with some cations (Li+, Na+, K+, Mg2+, Ca2+) and anions (F˗, Cl˗, Br˗). Struct Chem 29:129–137
Vijay D, Zipse H, Narahari Sastry G (2008) On the cooperativity of cation-π and hydrogen bonding interactions. J Phys Chem B 112:8863–8867
Li Q, Li W, Cheng J, Gong B, Sun J (2008) Effect of methyl group on the cooperativity between cation-π interaction and NH...O hydrogen bonding. J Mol Struct Theochem 867:107–110
Bertran J, Rodriguez-Santiago L, Sodupe M (1999) The different nature of bonding in Cu+-glycine and Cu2+-glycine. J Phys Chem B 103:2310–2317
Rodriguez-Santiago L, Sodupe M, Tortajada J (2001) Gas-phase reactivity of Ni+ with glycine. J Phys Chem A 105:5340–5347
Rogalewicz F, Ohanessian G, Gresh N (2000) Interaction of neutral and zwitterionic glycine with Zn2+ in gas phase: ab initio and SIBFA molecular mechanics calculations. J Comput Chem 21:963–973
Ai H, Bu Y, Li P, Li Z, Hu X, Chen Z (2005) Geometry and binding properties of different multiple-state glycine–Fe+/Fe2+ complexes. J Phys Org Chem 18:26–34
Rodgers MT, Armentrout PB (2004) A thermodynamic “vocabulary” for metal ion interactions in biological systems. Acc Chem Res 37:989–998
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford, CT, USA
Cohen AJ, Sanchez PM, Yang W (2008) Insights into current limitations of density functional theory. Science 321:792–794
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Iikura H, Tsuneda T, Yanai T, Hirao K (2001) A long-range correction scheme for generalized-gradient-approximation exchange functionals. J Chem Phys 115:3540
Savin A, Flad HJ (1995) Density functionals for the Yukawa electron-electron interaction. Int J Quantum Chem 56:327–332
Chai JD, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:084106
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the diferences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Bader RFW (1990) Atoms in Molecules: A Quantum Theory. Oxford University Press, New York
BieglerKönig F, Schönbohm J (2002) Update of the AIM2000-program for atoms in molecules. J Comput Chem 23:1489–1494
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Glendening ED, Reed AE, Carpenter JE, Weinhold F (2009) NBO, version 3.1 (in). Gaussian Inc, Pittsburg, CT
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20:129–154
Chattaraj PK, Poddar A (1999) Molecular reactivity in the ground and excited electronic states through density-dependent local and global reactivity parameters. J Phys Chem A 103:8691–8699
Parr RG, Lv S, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Sen KD, Jorgensen CK (1987) Electronegativity, Structure and Bonding. Springer Verlag, New York
Koopmans T (1933) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines atoms. Physica 1:104–113
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electrons densities. Chem Phys Lett 285:170–173
Palusiak M, Simon S, Sola M (2006) Interplay between intramolecular resonanceassisted hydrogen bonding and aromaticity in o-hydroxyaryl aldehydes. J Org Chem 71:5241–5248
Güell G, Poater J, Luis JM, Mó O, Yáñez M, Sola M (2005) Aromaticity analysis of Lithium Cation/π complexes of aromatic systems. Chem Phys Chem 6:2552–2561
Grabowski SJ, Sokalski WA, Dyguda E, Leszczyński J (2006) Quantitative classification of covalent and noncovalent H-bonds. J Phys Chem B 110:6444–6446
Dziembowska T (1990) Intramolecular Hydrogen Bonding. Akademia Rolnicza, Szczecin
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Parra RD, Ohlssen J (2008) Cooperativity in intramolecular bifurcated hydrogen bonds: an Ab initio study. J Phys Chem A 112:3492–3498
Ziółkowski M, Grabowski SJ, Leszczynski J (2006) Cooperativity in hydrogen-bonded interactions: ab initio and “atoms in molecules” analyses. J Phys Chem A 110:6514–6521
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Cremer D, Kraka E (1984) Chemical bonds without bonding electron density - does the difference electron-density analysis suffice for a description of the chemical bond? Angew Chem Int Ed Engl 23:627–628
Valinia F, Shojaei N, Ojaghloo P (2019) Novel 1-(4-chlorophenyl)-3-(2-ethoxyphenyl) triazene ligand: synthesis, X-ray crystallographic studies, spectroscopic characterization and DFT calculations. Chem Rev Lett 2:90–97
Fukui K (1982) Role of Frontier orbitals in chemical reactions. Science 218:747–754
Kosar B, Albayrak C (2011) Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim Acta A 78:160–167
Morell C, Labet V, Grand A, Chermette H (2009) Minimum electrophilicity principle: an analysis based upon the variation of both chemical potential and absolute hardness. Phys Chem Chem Phys 11:3417–3423
Majedi S, Behmagham F, Vakili M (2020) Theoretical view on interaction between boron nitride nanostructures and some drugs. J Chem Lett 1:19–24
Rauf HG, Majedi S, Mahmood EA, Sofi M (2019) Adsorption behavior of the Al- and Ga-doped B12N12 nanocages on COn (n=1,2) and HnX (n=2,3 and X=O, N): a comparative study. Chem Rev Lett 2:140–150
Mohamed RA, Adamu U, Sani U, Gideon SA, Yakub A (2019) Thermodynamics and kinetics of 1-fluoro-2-methoxypropane with Bromine monoxide radical (BrO•). Chem Rev Lett 2:107–117
Jalali Sarvestani MR, Ahmadi R, Farhang Rik B (2020) Procarbazine adsorption on the surface of single walled carbon nanotube: DFT studies. Chem Rev Lett 3:175–179
Hamer GK, Peat IR, Reynolds WF (1973) Investigations of substituent effects by nuclear magnetic resonance spectroscopy and all-valence electron molecular orbital calculations. II. 4-Substituted α-methylstyrenes and α-t-butylstyrenes. Can J Chem 51:915–926
Behmagham F, Asadi Z, Jamale Sadeghi Y (2018) Synthesis, spectroscopic and computational investigation of bis (3-methoxyphenylthio) ethyl) naphthalene. Chem Rev Lett 1:68–76
Acknowledgements
The support of this work by Vali-e-Asr University of Rafsanjan is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi, M., Alirezapour, F. & Khanmohammadi, A. DFT calculation of the interplay effects between cation–π and intramolecular hydrogen bond interactions of mesalazine drug with selected transition metal ions (Mn+, Fe2+, Co+, Ni2+, Cu+, Zn2+). Theor Chem Acc 140, 104 (2021). https://doi.org/10.1007/s00214-021-02813-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02813-1