Abstract
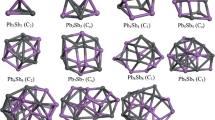
The particle swarm optimization method is used to search for the isomers of (BN)6 cluster, then the selected possible lower energy isomers are optimized using the DFT. Sixteen configurations that are stable stationary points on the potential energy surface at B3LYP/6-31G (d) level are found. Among the isomers, six of them have been reported and ten new low energy structures are found for the first time. We calculated the geometry optimization, infrared spectrum, relative Gibbs free energy topological analysis and polarizability. The lowest energy structure S1 is found to be the most stable configuration, which agrees with the previous results. By calculating Gibbs free energy, we also get their energy orders at different temperatures. The main interaction between B and N is demonstrated to be covalent by the topological analysis of (BN)6. Our calculated energy gap and polarizability obey the rule that a cluster with a wide energy gap always has small mean static polarizability.






Similar content being viewed by others
References
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Shirinyan AS, Gusak AM, Wautelet M (2005) Phase diagram versus diagram of solubility: What is the difference for nanosystems? Acta Mater 53:5025–5032
Hu JT, Odom TW, Lieber CM (1999) Chemistry and physics in one dimension: synthesis and properties of nanowires and nanotubes. Acc Chem Res 32:435–445
Stakheev AY, Kustov LM (1999) Effects of the support on the morphology and electronic properties of supported metal clusters: modern concepts and progress in 1990s. Appl Catal A 188:3–35
Daniel MC, Astruc D (2004) Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes—the route toward applications. Science 297:787–792
Taylor J, Guo H, Wang J (2001) Ab initio modeling of quantum transport properties of molecular electronic devices. Phys Rev B 63:245407
Kuno M, Oku T, Suganuma K (2001) Synthesis of boron nitride nanotubes and nanocapsules with LaB6. Diamond Relat Mater 10:1231–1234
Blase X, Rubio A, Louie SG, Cohen ML (1994) Stability and band gap constancy of boron nitride nanotubes. Europhys Lett 28:335–340
Chopra NG, Luyken RJ, Cherrey K, Crespi VH, Cohen ML, Louie SG, Zettl A (1995) Boron nitride nanotubes. Science 269:966–967
Golberg D, Bando Y, Eremets M, Takemura K, Kurashima K, Yusa H (1996) Nanotubes in boron nitride laser heated at high pressure. Appl Phys Lett 69:2045–2047
Iijima S (1980) High resolution electron microscopy of some carbonaceous materials. J Microsc 119:99–111
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Oku T, Kitahara H, Kuno M, Narita I, Suganuma K (2001) Synthesis, atomic structures and arrangement of carbon and boron nitride nanocage materials. Scr Mater 44:1557–1560
Huo KF, Hu Z, Chen F, Fu JJ, Chen Y, Liu BH, Ding J, Dong ZL, White T (2002) Synthesis of boron nitride nanowires. Appl Phys Lett 80:3611–3613
Sun ML, Slanina Z, Lee SL (1995) Square/hexagon route towards the boron-nitrogen clusters. Chem Phys Lett 233:279–283
Seifert G, Fowler PW, Mitchell D, Porezag D, Frauenheim T (1997) Boron-nitrogen analogues of the fullerenes: electronic and structural properties. Chem Phys Lett 268:352–358
Alexandre SS, Mazzoni MSC, Chacham H (1999) Stability, geometry, and electronic structure of the boron nitride B36N36 fullerene. Appl Phys Lett 75:61–63
Alexandre SS, Chacham H, Nunes RW (2001) Structure and energetics of boron nitride fullerenes: the role of stoichiometry. Phys Rev B 63:045402
Koi N, Oku T (2004) Hydrogen storage in boron nitride and carbon clusters studied by molecular orbital calculations. Solid State Commun 13:121–124
Koi N, Oku T (2004) Molecular orbital calculations of hydrogen storage in carbon and boron nitride clusters. Sci Technol Adv Mater 5:625–628
Oku T, Nishiwaki A, Narita I, Gonda M (2003) Formation and structure of B24N24 clusters. Chem Phys Lett 380:620–623
Martin JML, ElYazal J, Francois JP (1996) Structure and vibrations of BnNn (n=3–10). Chem Phys Lett 248:95–101
Stephan O, Bando Y, Loiseau A, Willaime F, Shramchenko N, Tamiya T, Sato T (1998) Formation of small single-layer and nested BN cages under electron irradiation of nanotubes and bulk material. Appl Phys A 67:107–111
Golberg D, Bando Y, Stephan O, Kurashima K (1998) Octahedral boron nitride fullerenes formed by electron beam irradiation. Appl Phys Lett 73:2441–2443
Wu HS, Jia JF (2004) Structures and stabilities of C24 and B12N12 clusters. Chinese J Struct Chem 23:580–585
Wu HS, Xu XH, Jia JF (2004) Structures and stabilities of B24N24 cage clusters. Acta Chim Sinica 62:28–33
Zhao Y-Q, Liu L, Hu C-E, Cheng Y (2018) Ab initio investigation of structure, stability, thermal behavior and infrared spectra of (BN)4 cluster. Comput Theor Chem 1141:1–6
Strout DL (2000) Structure and stability of boron nitrides: isomers of B12N12. J Phys Chem A 104:3364–3366
Matxain JM, Ugalde JM, Towler MD, Needs RJ (2003) Stability and arornaticity of BiNi rings and fullerenes. J Phys Chem A 107:10004–10010
Jia JF, Wu HS, Jiao HJ (2003) Structure and stability of (BN)n clusters. Acta Chim Sin 61:653–659
Nirmala V, Kolandaivel P (2005) Post Hartree-Fock and density functional theory studies on (BN)n=1–10 clusters. Int J Nanosci 04:377–388
Xu SH, Zhang MY, Zhao YY, Chen BG, Zhang J, Sun CC (2006) Stability and property of planar (BN)x clusters. Chem Phys Lett 423:212–214
Song Y, Chen HS, Zhang CR, Wang GH (2005) Structures and bonding properties of (BN)(n) (n<=12) clusters. Acta Phys Chim Sin 21:735–739
Wang Y, Lv J, Zhu L, Ma Y (2010) Crystal structure prediction via particle-swarm optimization. Phys Rev B 82:094116
Frisch MJ Trucks GW Schlegel HB et al. (2009) GAUSSIAN 09, Revision B. 01. Gaussian Inc., Wallingford, CT.
Lv ZL, Cheng Y, Chen XR, Cai LC (2015) Structural exploration and properties of (H2O)4+ cluster via ab initio in combination with particle swarm optimization method. Chem Phys 452:25–30
Lv ZL, Xu K, Cheng Y, Chen XR, Cai LC (2014) Ab initio investigation of the lower energy candidate structures for (H2O)5+ water cluster. J Chem Phys 141:054309
Lv J, Wang Y, Zhu L, Ma Y (2014) B38: an all-boron fullerene analogue. Nanoscale 6:11692–11696
Becke AD (1988) Density-functional exchange-enegry approximation with correct asymptotic-behavior. Phys Rev A 38:3098–3100
Lee CT, Yang WT, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Merrick JP, Moran D, Radom L (2007) An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A 111:11683–11700
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Guerini S, Piquini P (2003) Structural, electronic, and vibrational properties of BxNy (x+y=6) clusters. Int J Quantum Chem 95:329–335
Singh NJ, Park M, Min SK, Suh SB, Kim KS (2006) Magic and antimagic protonated water clusters: exotic structures with unusual dynamic effects. Angew Chem Int Ed 45:3795–3800
Cioslowski J (1991) Atoms in molecules: a quantum theory. Science 252:1566–1567
Parthasarathi R, Subramanian V, Sathyamurthy N (2006) Hydrogen bonding without borders: an atoms-in-molecules perspective. J Phys Chem A 110:3349–3351
Matta CF, Bader RFW (2000) An atoms-in-molecules study of the genetically-encoded amino acids: i. effects of conformation and of tautomerization on geometric, atomic, and bond properties. Proteins Struct Funct Bioinform 40:310–329
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. WIREs Comput Mol Sci 2:1–42
Minkin VI, Glukhovtsev MN, Simkin BY (1988) σ-Aromaticity and σ-antiaromaticity. J Mol Struct Theochem 50:93–110
Perez-Juste I, Mandado M, Carballeira L (2010) Contributions from orbital-orbital interactions to nucleus-independent chemical shifts and their relation with aromaticity or antiaromaticity of conjugated molecules. Chem Phys Lett 491:224–229
Karadakov PB, Horner KE (2013) Magnetic shielding in and around benzene and cyclobutadiene: a source of information about aromaticity, antiaromaticity, and chemical bonding. J Phys Chem A 117:518–523
Schleyer PV, Maerker C, Dransfeld A, Jiao HJ, Hommes N (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Schleyer PV, Manoharan M, Wang ZX, Kiran B, Jiao HJ, Puchta R, Hommes N (2001) Dissected nucleus-independent chemical shift analysis of pi-aromaticity and antiaromaticity. Org Lett 3:2465–2468
Fallah-Bagher-Shaidaei H, Wannere CS, Corminboeuf C, Puchta R, Schleyer PV (2006) Which NICS aromaticity index for planar pi rings is best? Org Lett 8:863–866
Arrué L, Pino-Rios R (2020) Revisiting (anti)aromaticity and chemical bond in planar BxNx clusters (x=2-11). Int J Quantum Chem 120:e26403
Guliamov O, Kronik L, Martin JML (2007) Polarizability of small carbon cluster anions from first principles. J Phys Chem A 111:2028–2032
Namazian M, Orangi N, Noorbala MR (2014) Thermodynamic stability and structural parameters of carbon nanoclusters. J Theor Comput Chem 13:1450058
Buckingham AD (2014) Communication: permanent dipoles contribute to electric polarization in chiral NMR spectra. J Chem Phys 140:011103
Hohm U (2000) Is there a minimum polarizability principle in chemical reactions? J Phys Chem A 104:8418–8423
Parr RG, Chattaraj PK (1991) Principle of maximun hardness. J Am Chem Soc 113:1854–1855
Shevlin SA, Guo ZX, van Dam HJJ, Sherwood P, Catlow CRA, Sokol AA, Woodley SM (2008) Structure, optical properties and defects in nitride (III–V) nanoscale cage clusters. Phys Chem Chem Phys 10:1944–1959
Acknowledgements
The authors are grateful for the supports of the NSAF (Grant No. U1830101) and the National Natural Science Foundation of China (Grant No. 11504035).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, YQ., Cheng, Y., Hu, CE. et al. Structural exploration and properties of (BN)6 cluster via ab initio in combination with particle swarm optimization method. Theor Chem Acc 140, 51 (2021). https://doi.org/10.1007/s00214-021-02759-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02759-4