Abstract
Summary
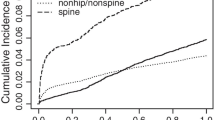
We evaluated healthcare utilization associated with treating fracture types in >51,000 women aged ≥55 years. Over the course of 1 year, there were five times more non-hip, non-spine fractures than hip or spine fractures, resulting in twice as many days of hospitalization and rehabilitation/nursing home care for non-hip, non-spine fractures.
Introduction
The purpose of this study is to evaluate medical healthcare utilization associated with treating several types of fractures in women ≥55 years from various geographic regions.
Methods
Information from the Global Longitudinal Study of Osteoporosis in Women (GLOW) was collected via self-administered patient questionnaires at baseline and year 1 (n = 51,491). Self-reported clinically recognized low-trauma fractures at year 1 were classified as incident spine, hip, wrist/hand, arm/shoulder, pelvis, rib, leg, and other fractures. Healthcare utilization data were self-reported and included whether the fracture was treated at a doctor’s office/clinic or at a hospital. Patients were asked if they had undergone surgery or been treated at a rehabilitation center or nursing home.
Results
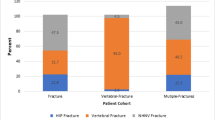
During 1-year follow-up, there were 195 spine, 134 hip, and 1,654 non-hip, non-spine fractures. Clinical vertebral fractures resulted in 617 days of hospitalization and 512 days of rehabilitation/nursing home care; hip fractures accounted for 1,306 days of hospitalization and 1,650 days of rehabilitation/nursing home care. Non-hip, non-spine fractures resulted in 3,805 days in hospital and 5,186 days of rehabilitation/nursing home care.
Conclusions
While hip and vertebral fractures are well recognized for their associated increase in health resource utilization, non-hip, non-spine fractures, by virtue of their 5-fold greater number, require significantly more healthcare resources.

Similar content being viewed by others
References
No Author (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650
Melton LJ 3rd (1988) Epidemiology of fractures. In: Riggs BL, Melton LJ 3rd (eds) Osteoporosis: etiology, diagnosis and management. Raven, New York, pp 135–155
Melton LJ, 3rd, Sampson JM, Morrey BF, Ilstrup DM (1981) Epidemiologic features of pelvic fractures. Clin Orthop Relat Res 43–47
Nilsson BE (1969) Age and sex incidence of ankle fractures. Acta Orthop Scand 40:122–129
Rose SH, Melton LJ, 3rd, Morrey BF, Ilstrup DM, Riggs BL (1982) Epidemiologic features of humeral fractures. Clin Orthop Relat Res 24–30
Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, Cummings SR (1991) Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med 115:837–842
Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL (1992) Perspective. How many women have osteoporosis? J Bone Miner Res 7:1005–1010
Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16:229–238
Cooper C (1999) Epidemiology of osteoporosis. Osteoporos Int 9(Suppl 2):S2–S8
Adachi JD, Adami S, Gehlbach S, Anderson FA Jr, Boonen S, Chapurlat RD, Compston JE, Cooper C, Delmas P, Diez-Perez A, Greenspan SL, Hooven FH, LaCroix AZ, Lindsay R, Netelenbos JC, Wu O, Pfeilschifter J, Roux C, Saag KG, Sambrook PN, Silverman S, Siris ES, Nika G, Watts NB (2010) Impact of prevalent fractures on quality of life: baseline results from the Global Longitudinal study of Osteoporosis in Women. Mayo Clin Proc 85:806–813
Adachi JD, Ioannidis G, Pickard L, Berger C, Prior JC, Joseph L, Hanley DA, Olszynski WP, Murray TM, Anastassiades T, Hopman W, Brown JP, Kirkland S, Joyce C, Papaioannou A, Poliquin S, Tenenhouse A, Papadimitropoulos EA (2003) The association between osteoporotic fractures and health-related quality of life as measured by the Health Utilities Index in the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 14:895–904
Ioannidis G, Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pickard L, Kennedy CC, Prior JC, Olszynski WP, Davison KS, Goltzman D, Thabane L, Gafni A, Papadimitropoulos EA, Brown JP, Josse RG, Hanley DA, Adachi JD (2009) Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 181:265–271
Lindsay R (1995) The burden of osteoporosis: cost. Am J Med 98:9S–11S
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2:285–289
Gullberg B, Johnell O, Kanis JA (1997) World-wide projections for hip fracture. Osteoporos Int 7:407–413
Melton LJ 3rd (1993) Hip fractures: a worldwide problem today and tomorrow. Bone 14(Suppl 1):S1–S8
Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L (1992) Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 305:160–164
Brooks R (1996) EuroQol: the current state of play. Health Pol 37:53–72
Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey. NHANES 2005–2006: National Center for Health Statistics; 2008. http://www.cdc.gov/nchs/nhanes.htm. Accessed 28 March 2011
Knowelden J, Buhr AJ, Dunbar O (1964) Incidence of fractures in persons over 35 years of age. A report to the M.R.C. Working Party on Fractures in the Elderly. Br J Prev Soc Med 18:130–141
Cooper C, O'Neill T, Silman A (1993) The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group. Bone 14(Suppl 1):S89–S97
Dolan P, Torgerson DJ (1998) The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int 8:611–617
Finnern HW, Sykes DP (2003) The hospital cost of vertebral fractures in the EU: estimates using national datasets. Osteoporos Int 14:429–436
Kanis JA, McCloskey EV (1992) Epidemiology of vertebral osteoporosis. Bone 13(Suppl 2):S1–S10
Garraway WM, Stauffer RN, Kurland LT, O'Fallon WM (1979) Limb fractures in a defined population. II. Orthopedic treatment and utilization of health care. Mayo Clin Proc 54:708–713
Kanis JA, Pitt FA (1992) Epidemiology of osteoporosis. Bone 13(Suppl 1):S7–S15
Schwenkglenks M, Lippuner K, Hauselmann HJ, Szucs TD (2005) A model of osteoporosis impact in Switzerland 2000–2020. Osteoporos Int 16:659–671
Bouza C, Lopez T, Palma M, Amate JM (2007) Hospitalised osteoporotic vertebral fractures in Spain: analysis of the national hospital discharge registry. Osteoporos Int 18:649–657
Phillips S, Fox N, Jacobs J, Wright WE (1988) The direct medical costs of osteoporosis for American women aged 45 and older, 1986. Bone 9:271–279
Lippuner K, von Overbeck J, Perrelet R, Bosshard H, Jaeger P (1997) Incidence and direct medical costs of hospitalizations due to osteoporotic fractures in Switzerland. Osteoporos Int 7:414–425
King AB, Tosteson AN, Wong JB, Solomon DH, Burge RT, Dawson-Hughes B (2009) Interstate variation in the burden of fragility fractures. J Bone Miner Res 24:681–692
Johnell O, Gullberg B, Kanis JA (1997) The hospital burden of vertebral fracture in Europe: a study of national register sources. Osteoporos Int 7:138–144
Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C (2005) The burden of hospitalised fractures in Sweden. Osteoporos Int 16:222–228
Hooven FH, Adachi JD, Adami S, Boonen S, Compston J, Cooper C, Delmas P, Diez-Perez A, Gehlbach S, Greenspan SL, LaCroix A, Lindsay R, Netelenbos JC, Pfeilschifter J, Roux C, Saag KG, Sambrook P, Silverman S, Siris E, Watts NB, Anderson FA Jr (2009) The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int 20:1107–1116
Acknowledgment
We thank the physicians and project coordinators participating in GLOW. Editorial support for the final version of this article, comprising language editing, content checking, formatting and referencing was provided by Sophie Rushton-Smith, PhD. Dr Boonen is senior clinical investigator of the Fund for Scientific Research, Flanders, Belgium (FWO-Vlaanderen) and holder of the Leuven University Chair in Metabolic Bone Diseases. Financial support for the GLOW study is provided by Warner Chilcott Company, LLC and Sanofi–Aventis to the Center for Outcomes Research, University of Massachusetts Medical School. The sponsor had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflicts of interest
George Ioannidis: None.
Julie Flahive: The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott).
Laura Pickard: None.
A Papaioannou: Consultant/Speaker, Honoraria, or Grants: Eli Lilly, Amgen, Merck, Novartis, Procter & Gamble, sanofi-aventis, and Servier.
Roland Chapurlat: French Ministry of Health, Merck, Servier, Lilly, Procter and Gamble. Speakers’ Bureau: none. Honoraria: Amgen, Servier, Novartis, Lilly, Roche, sanofi-aventis. Consultant/Advisory Board: Amgen, Merck, Servier, Nycomed, Novartis.
Kenneth G Saag: Speakers’ bureau: Novartis. Consulting fees or other remuneration: Eli Lilly & Co., Merck, Novartis, Amgen, Roche, Proctor & Gamble, sanofi-aventis. Paid research: Eli Lilly & Co, Merck, Novartis, Amgen, Proctor & Gamble, sanofi-aventis. Advisory Committee or other paid committee: Eli Lily & Co.
Stuart Silverman: Research grants: Wyeth, Lilly, Novartis, Alliance. Speakers’ bureau: Lilly, Novartis, Pfizer, Procter & Gamble. Honoraria: Procter & Gamble. Consultant/Advisory Board: Lilly, Argen, Wyeth, Merck, Roche, Novartis.
Frederick A Anderson has received research support from sanofi-aventis, The Medicines Company, Procter & Gamble, and Scios; has been a consultant for sanofi-aventis, GlaxoSmithKline, and Millennium and Sage; and has served on advisory boards for sanofi-aventis and The Medicines Company. Stephen Gehlbach: The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott).
Frederick H Hooven: The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott).
Steven Boonen: Research grant: Amgen, Eli Lilly, Novartis, Pfizer, Procter & Gamble, sanofi-aventis, Roche, GlaxoSmithKline. Speakers’ bureau: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, sanofi-aventis, Servier. Honoraria: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, sanofi-aventis, Servier. Consultant/Advisory Board: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, sanofi-aventis, Servier.
Juliet Compston: Paid consultancy work: Servier, Shire, Nycomed, Novartis, Amgen, Procter & Gamble, Wyeth, Pfizer, Alliance for Better Bone Health, Roche, GlaxoSmithKline. Paid speaking engagements, reimbursement, and travel and accommodation: Servier, Procter & Gamble, Eli Lilly. Research grants from Servier R&D and Procter & Gamble. No stocks or shares in relevant companies.
Cyrus Cooper: has received consulting fees and lectured for AMGEN, The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott), Eli Lily, Merck Sharp and Dohme, Servier, Novartis, and Roche-GSK.
Adolfo Diez-Perez: Honoraria: Novartis, Eli Lilly, Amgen, Procter & Gamble, Roche. Expert Witness: Merck. Consultant/Advisory board: Novartis, Eli Lilly, Amgen, Procter & Gamble.
Susan L Greenspan: Consultant/advisory board: Amgen, Lilly, Merck. Research grants: The Alliance for Better Bone Health (sanofi-aventis and Proctor & Gamble), Lilly.
Andrea LaCroix: The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott).
Robert Lindsay: The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott).
J Coen Netelenbos: Paid consultancy work: Roche Diagnostics, Daiichi-Sankyo, Proctor & Gamble, Nycomed. Paid speaking engagements, reimbursement and travel and accommodation: Roche Diagnostics, Novartis, Daiichi-Sankyo, Procter & Gamble. Research grants: Alliance for Better Bone Health, Amgen.
Johannes Pfeilschifter: Research grant: AMGEN, Kyphon, Novartis, Roche. Other research support. Equipment: GE LUNAR. Speakers’ bureau: AMGEN, sanofi-aventis, GlaxoSmithKline, Roche, Lilly Deutschland, Orion Pharma, Merck Sharp and Dohme, Merckle, Nycomed, Procter & Gamble. Advisory Board membership: Novartis, Roche, Procter & Gamble, TEVA.
Maurizio Rossini: Speaker: Roche, Merck Sharp & Dohme, GlaxoSmithKline.
Christian Roux: Honoraria: Alliance, Amgen, Lilly, Merck Sharp and Dohme, Novartis, Nycomed, Roche, GlaxoSmithKline, Servier, Wyeth. Consultant/Advisory board: Alliance, Amgen, Lilly, Merck Sharp and Dohme, Novartis, Nycomed, Roche, GlaxoSmithKline, Servier, Wyeth.
Philip N Sambrook: Honoraria: Merck, sanofi-aventis, Roche, Servier. Consultant/Advisory board: Merck, sanofi-aventis, Roche, Servier.
Ethel S Siris: Consultant: Amgen, Lilly, Novartis, and the Alliance for Better Bone Health. Speakers’ Bureau: Amgen, Lilly. Nelson B Watts: Stock options/holdings, royalties, company owner, patent owner, official role: none. Honoraria for lectures in past year: Amgen, Novartis, Procter & Gamble, sanofi-aventis. Consulting in past year: Amgen, Baxter, InteKrin, Johnson & Johnson, MannKind, Novo Nordisk, NPS, Pfizer, Procter & Gamble, sanofi-aventis, Takeda Pharmaceuticals, Warner Chilcott. Research support (through University): Amgen, Eli Lilly, Merck, NPS.
Jonathan D Adachi: Consultant/Speaker: Amgen, Astra Zeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Servier, Wyeth and Bristol-Myers Squibb. Clinical trials for Amgen, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Wyeth and Bristol-Myers Squibb. Stock: nothing to declare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ioannidis, G., Flahive, J., Pickard, L. et al. Non-hip, non-spine fractures drive healthcare utilization following a fracture: the Global Longitudinal Study of Osteoporosis in Women (GLOW). Osteoporos Int 24, 59–67 (2013). https://doi.org/10.1007/s00198-012-1968-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-1968-z