Abstract
Objectives
To assess the reproducibility of respiratory dead space measurements in ventilated children.
Design
Prospective study.
Setting
University pediatric intensive care unit.
Patients
Thirty-two mechanically ventilated children (0.13–15.4 years) who were clinically stable.
Methods
The single-breath CO2 test (SBT-CO2) was recorded using the CO2SMO Plus from the mean of 30 ventilatory cycles during 1 h (at T0, T15, T30, T45, and T60). Airway dead space was determined automatically (Novametrix Medical Systems, USA), and manually by Bohr- Enghoff equations using data obtained by SBT-CO2. At the end of the study period, arterial blood gas was sampled in order to calculate alveolar and physiologic dead space. Intrasubject reproducibility of measurements was evaluated by the intraclass correlation coefficient. Two-way analysis of variance was used to evaluate the relationships between time and measurements. The two methods for calculating airway dead space were compared by using two-tailed Student’s t-test and Bland-Altman analysis.
Results
Airway dead space measurement had a good reproducibility during the 1-h period, whatever the method used (intraclass correlation coefficient: 0.84 to 0.87). No significant difference was observed with time. Airway dead space values from the SBT-CO2 method were smaller than those from Bohr-Enghoff equations. Physiologic dead space values from the SBT-CO2 method were similar to those from Bohr-Enghoff equations.
Conclusion
The measurement of airway dead space by the CO2SMO Plus was reproducible over a 1-h period in children requiring mechanical ventilation, provided ventilatory parameters were constant throughout the study. SBT-CO2 analysis may provide a bedside non-invasive monitoring of volumetric capnography.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary dead space (VD) represents the portion of the respiratory system not involved in gas exchange and includes both alveolar dead space and airway dead space. Airway (or anatomic) dead space (VDaw), equals the volume of conducting airways. Alveolar dead space (VDalv) is caused by ventilated but not perfused areas. In conditions without ventilation/perfusion inequalities, there would be no VDalv [1]. Physiologic dead space (VDphysiol) equals VDalv plus VDaw, and is often expressed as a ratio to tidal volume (VD/VT). During the past two decades, this ratio has been used to identify survivability of infants with congenital diaphragmatic hernia [2], detect pulmonary shunts in congenital heart children [3], determine pulmonary improvements in neonates with extracorporeal membrane oxygenation [4], and predict successful extubation in infants and children [5]. Initially, VD/VT was measured by collecting expired gas. Recent advances in computer and capnography technology have provided a simplified and automated method for calculating VD/VT from single-breath CO2 waveforms (SBT-CO2) [6, 7, 8, 9, 10]. Besides the above-mentioned conditions in which VD/VT has been considered as useful, there is very little information concerning the reproducibility of VD measurements in the pediatric ICU [11].
The main objective of this study was to evaluate in ventilated children the reproducibility of VD measurements by SBT-CO2 test using the CO2SMO Plus over a 1-h period (automatic measurement by the software and manual calculation according to Bohr-Enghoff equations).
The secondary objective was to compare calculation of VD by SBT-CO2 to that obtained by Bohr-Enghoff equations, since discrepancies between the two methods were previously reported [3].
Patients and methods
Patient population
During the study period (January 2002 to June 2002), of 105 children hospitalized in the pediatric ICU, 51 were mechanically ventilated and 32 fulfilled the protocol inclusion criteria: pressure-controlled mechanical ventilation (servo 900 or 300C ventilator, Siemens-Elema, Solna, Sweden), stable haemodynamic and respiratory conditions 1 h before and during the measurements, no suctioning and no changes in ventilator settings, sedation, and therapeutics during the study period. The local Ethics Committee approved this study.
Measurement of pulmonary dead space and respiratory mechanics
VD and respiratory mechanics measurements were calculated from the mean of 30 ventilatory cycles, at each 15-min period, during 1 h (T0, T15, T30, T45, and T60). At the end of the study period, arterial blood gas was sampled. At each time period, haemodynamic parameters (heart rate, blood pressure, and SpO2) and ventilator settings [peak inspiratory pressure (PIP), positive end-expiratory pressure (PEEP), mean airway pressure (Paw), inspiratory time (Ti), expiratory time (Te), and inspired oxygen concentration (FiO2)] were obtained.
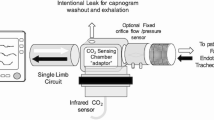
VD measurements were performed using the CO2SMO Plus and its computer software. Among the different capnographs, the CO2SMO Plus (Novametrix Medical Systems, Wallingford, Conn., USA) is suitable for bedside VD measurements in children and incorporates mainstream capnograph and gas flow monitor via a dual-purpose sensor in connection with a computer which enables automatic calculation of over 50 parameters on each breath (Analysis Plus! for Windows program, version 2.0, Novametrix Medical Systems). This device has been previously demonstrated to be accurate by Arnold et al., who measured VDaw in a lung model and quantified the bias and precision of VDphysiol measurement in a surfactant-depleted animal model [9]. Its pediatric sensor (combined CO2/Flow sensor series 3, VD less than 4 ml) was inserted between the Y-piece and the endotracheal tube without any additional connector. Flow is measured with this device (flow range: 0.5–120 l/min, tracheal tube internal diameter: 3.5–6.0 mm, tidal volume: 30–400 ml). Airway and barometric pressure are measured with absolute pressure transducers and airway pressure is defined relative to the barometric pressure. CO2 is measured by a mainstream infrared absorption technique (response time less than 75 ms, accuracy: ±2 mmHg from 0 mmHg to 40 mmHg; 5% reading from 41 mmHg to 100 mmHg). The CO2SMO Plus provides a standard expired SBT-CO2 waveform that can be divided in three phases [6]. In phase I, no CO2 is found. It corresponds to the “absolute VD” from conducting airways. In phase II, the content of expired CO2 increases because the expired gas contains mixed air from the alveoli and the airways. The third phase has a slightly increased CO2 concentration which represents air from alveoli. By knowing the end-tidal (PETCO2) and arterial (PaCO2) CO2 concentrations and the expired volumes during the three phases, VDphysiol, VDaw, and VDalv can be calculated by using Aitken and Clarke-Kennedy’s principle [6]. Briefly, the measurement offered by the software is based on an automatic recognition of the areas X, Y, Z generated by the shape of the PCO2/volume curve in phases I, II, and III. (Fig. 1):\(\begin{array}{*{20}l} {{{\text{V}}_{{{\text{Daw}}}} /{\text{VT}} = {\text{Z}}/{\left( {{\text{X}} + {\text{Y}} + {\text{Z}}} \right)}} \hfill} \\ {{{\text{V}}_{{{\text{Dalv}}}} {\text{/VT}} = {\text{Y}}/{\left( {{\text{X}} + {\text{Y}} + {\text{Z}}} \right)}} \hfill} \\ {{{\text{V}}_{{{\text{Dphysiol}}}} /{\text{VT}} = {\left( {{\text{Y}} + {\text{Z}}} \right)}/{\left( {{\text{X}} + {\text{Y}} + {\text{Z}}} \right)}} \hfill} \\ \end{array} \)
Airway dead space (VDaw) as illustrated by a CO2-volume plot (SBT-CO2). Triangles p and q are of equal area. Area X is the volume of CO2 in the breath (VCO2), while areas Z and Y are defects in CO2 elimination which represent wasted ventilation due to VDaw and alveolar dead space (VDalv) respectively. Alveolar tidal volume (VTalv) represents difference between tidal volume (VT) and airway dead space (VDaw) [8]
Area X is the volume of CO2 in the breath (VCO2), which allows us to calculate CO2 output (V’CO2=VCO2 x respiratory rate). The slope of phase III is computed by linear regression of the points bounded by 30% to 70% of expired volume and is extrapolated to determine the end point of mixed air (phase II) and the beginning of alveolar volume exhalation (phase III). Then, a vertical line is set in the middle of phase II so that areas p and q are equal (Fig. 1).
VD can be also manually determined by using Bohr-Enghoff equations [12, 13]:\(\begin{array}{*{20}l} {{{\text{V}}_{{{\text{Daw}}}} = {\text{VT}}_{{\text{E}}} x{\left( {{\left( {{\text{P}}_{{{\text{ET}}}} {\text{CO}}_{2} - {\text{P}}_{{\text{e}}} {\text{CO}}_{2} } \right)}/{\left( {{\text{P}}_{{{\text{ET}}}} {\text{CO}}_{2} - {\text{P}}_{{\text{i}}} {\text{CO}}_{2} } \right)}} \right)}} \hfill} \\ {{{\text{V}}_{{{\text{Dphysiol}}}} = {\text{VT}}_{{\text{E}}} x{\left( {{\left( {{\text{P}}_{{\text{a}}} {\text{CO}}_{2} - {\text{P}}_{{\text{e}}} {\text{CO}}_{2} } \right)}/{\left( {{\text{P}}_{{\text{a}}} {\text{CO}}_{2} - {\text{P}}_{{\text{i}}} {\text{CO}}_{2} } \right)}} \right)}} \hfill} \\ {{{\text{V}}_{{{\text{Dalv}}}} = {\text{V}}_{{{\text{Dphysiol}}}} - {\text{V}}_{{{\text{Daw}}}} } \hfill} \\ \end{array} \) where VTE represents the expired tidal volume, PeCO2 mixed expired PCO2, and PiCO2 mixed inspired PCO2. All these parameters, except PaCO2, were calculated by the CO2SMO Plus. The apparatus VD of the sensor was automatically subtracted from the measured VD. CO2 sensor was systematically calibrated between each time period.
Respiratory mechanics data collection included Paw, PIP, PEEP, intrinsic positive end-expiratory pressure PEEPi, dynamic compliance of the respiratory system, inspiratory and expiratory airway resistance, and inspired tidal volume VTI and VTE. Airleak from around the endotracheal tube is computed as [(VTE-VTI)/VTI]. VT and VD are normalized to patient weight.
Statistical analysis
Statistical analysis was performed with SPSS 11.0 (SPSS, Chicago, Ill., USA). The results are expressed as mean±SD. Intrasubject reproducibility of measurements repeated over 1 h was evaluated by the intraclass correlation coefficient (ICC). Considering the ICC value, the reproducibility was qualified as excellent (≥0.81), good (0.80–0.70) or bad (≤0.70) [14]. Two-way analysis of variance was used to evaluate the relationships between time and measurements.
The two methods for calculating VDaw from T0 to T60 and the three different components of VD obtained by the two methods at T60 were compared by two-tailed Student’s t-test and Bland and Altman analysis after modelling the variability in the SD of the differences as a function of the level of measurement when required [15]. Differences were considered significant with P<0.05.
Results
Patients
Thirty-two children ranging from 0.13 years to 15.4 years were enrolled (median age: 1.91 years; median weight: 13 kg). Fourteen patients had primary lung disease such as pneumonia (n = 11), sepsis (n = 2), and acute respiratory disease (n = 1). Twelve patients had neurologic disease such as encephalopathy (n = 4), status epilepticus (n = 3), meningitis (n = 2), neuromuscular disease (n = 2), and head trauma (n = 1). Six patients had cardiovascular disease. Ventilator settings, and respiratory and haemodynamic parameters remained unchanged during the study period (Table 1). No significant changes in SpO2 or heart rate with time were observed.
Reproducibility of primary parameters and airway dead space measurements obtained with the SBT-CO2 and the Bohr-Enghoff equations over a 1-h period
Table 2 shows that PiCO2 increased significantly with time (P=0.02; ICC=0.77). Interestingly, PiCO2 values were significantly higher (P<0.03) in infants weighting less than 5 kg (n = 8) compared to those in infants weighting more than 15 kg (n = 11) whatever the time period (1.24±3.03 mmHg, 2.67±5.31 mmHg, 3.22±4.24 mmHg, 4.15±3.70 mmHg, 4.04±4.64 mmHg vs 0±0 mmHg, 1.79±3.77 mmHg, 2.18±4.61 mmHg, 2.64±6.00 mmHg, and 3.00±6.86 mmHg, respectively, at T0, T15, T30, T45, and T60). Consequently, PETCO2 values were corrected according to this PiCO2 increase with time, such as PETCO2 corr = (PETCO2−PiCO2). VDaw obtained from Bohr equation after correction for the increase in PiCO2 with time were significantly smaller than non-corrected VDaw (mean difference: 0.187±0.363 ml/kg, or 5.01% of mean value, P<0.001). The 95% confidence interval (CI) for this bias was –0.126 ml/kg to 0.250 ml/kg. The limits of agreement were −0.539 ml/kg to 0.915 ml/kg. Table 2 shows that VDaw measurement had a good reproducibility during the 1-h period, whatever the method used [ICC ranged from 0.84 to 0.87, within-subject coefficient of variation (CV) for VDaw and VDaw/VT was, respectively, for SBT-CO2 test: 9.9 and 11.4%, and for Bohr-Enghoff equations: 8.4 and 6.2%]. No significant differences in measurement were observed with time. No significant relation was observed between age or weight and VD values (r=0.4).
Comparison of the dead space components calculation using SBT-CO2 test and that using Bohr-Enghoff equations.
Table 2 shows that, from T0 to T60, VDaw calculated from SBT-CO2 method were always smaller than those obtained from Bohr-Enghoff equations. The mean percent difference for the VDaw calculated from the SBT-CO2 analysis was 81.1% of mean value (the mean percent difference was calculated from the following formula: percent difference (%) = 100 × (difference between the methods)/mean dead space measurement). Bland and Altman analysis revealed a non-uniform relationship between the VDaw difference and the magnitude. The difference between the VDaw obtained by the two methods was regressed on their average (x). The regression equation was y = −0.101–0.790x. The variability of the differences increased as the magnitude of the differences increased. The SD of the residuals was modeled as a function of the magnitude of VTE to obtain the limits of agreement [15].
Table 3 shows that VDaw and VDalv calculated at T60 from the two methods appeared different even after correction for the PiCO2 (P<0.01). Bland and Altman analysis revealed a non-uniform relationship between the VDaw difference and the magnitude as shown in Fig. 2. The difference between the VDaw obtained by the two methods was regressed on their average (x). The regression equation was y = −0.203–0.760x. The variability of the differences increased as the magnitude of the differences increased. The SD of the residuals was modeled as a function of the magnitude of VTE to obtain the limits of agreement [15].
VDphysiol obtained from SBT-CO2 method was quite similar to VDphysiol calculated from Bohr-Enghoff equations as shown in Fig. 2, (mean difference: −0.287±1.046 ml/kg, or 6.8% of mean value, NS). The 95% CI for the bias was −0.690 ml/kg to 0.116 ml/kg. The limits of agreement were −1.806 ml/kg to 2.380 ml/kg.
Discussion
This study demonstrated that the measurement of VDaw by the CO2SMO Plus was reproducible over a 1-h period, in ventilated children, provided ventilatory parameters were kept constant throughout the study. To our knowledge, this is the first clinical study evaluating in children the reproducibility of SBT-CO2 analysis. In adults too, the data seem very scarce: Koulouris et al. [16] found that the mean within-study, within-day, and day per day CV for VDaw/VT calculated from Bohr’s equation was 6.5%, 6.8%, and 7.25% in three normal adults in whom measurements were repeated three times per day for three consecutive days. In our study, the within-subject CV for VDaw and VDaw/VT was comparable (SBT-CO2: 9.9% and 11.4%, Bohr-Enghoff equations: 8.4% and 6.2%).
A wide range of VDaw values was observed in our patients, whatever the method used (SBT-CO2: 0.81–4.20 ml/kg; Bohr equation: 1.48–5.90 ml/kg). These results can be compared to data from the literature in ventilated infants (VDaw ranges from 1.6 ml/kg to 3.2 ml/kg) [10]. In our patients, variability could be explained by the heterogeneity of the population and the wide range of ages (0.13–15.4 years). In fact, Numa et al. demonstrated in 40 patients aged 7 days to 14.2 years who were intubated with cuffed endotracheal tubes, that extrathoracic VDaw decreased exponentially with increasing age, ranging from 2.3 ml/kg in early infancy to 0.8 ml/kg in children older than 6 years. Mean intrathoracic VDaw was 1.03 ml/kg and was not related to age [17]. However, in our study, no significant relation was observed between age or weight and dead space values.
Although major difficulties of this technique are eliminated when applied to intubated infants, a zeroing of the PCO2-volume plot at the starting point of expiration and an alveolar plateau of the PCO2-volume plots are necessary to apply SBT-CO2 [18]. In our study, PiCO2 slightly increased with time and this was probably due to rebreathing of expired gas, because of the pediatric sensor VD. In fact, PiCO2 values were significantly higher in small infants. This was also observed by Wenzel et al. who tested the applicability of Ventrak 1550/Capnogard 265 for VD measurement in 22 ventilated neonates: after inserting the combined sensor of the device, transcutaneous PCO2 rose within 5 min by 3.2% in newborns of ≥2,500 g and by 5.7% in those of <2,500 g [10]. Rebreathing of expired gas detected by an increased end-inspiratory CO2 impairs alveolar gas exchange [19] which can lead to an overestimation of PETCO2 and PeCO2. SBT-CO2 enables the calculation of PiCO2, defined as the minimum value of the moving-average of the CO2 sample over the last 20 s, and the correction of CO2 elimination and PeCO2 (calculated by dividing VCO2 by VT) for rebreathed PiCO2. Conversely, simplified Bohr-Enghoff equations do not take into account rebreathed PiCO2 and may overestimate VDaw values [20]. Fletcher et al. estimated that rebreathing was the most important source of error in the measurement of CO2 elimination. By using a 3-l rubber bag containing radioactive xenon and connected to a ventilator, they demonstrated that the calculated rebreathed volume corresponded to about 24 ml of end-expiratory gas per breath with a standard Y-piece and tubing, at a frequency of 10 bpm and an overestimation of FeCO2 by 5–12% [19]. These results were similar to those of our study in which the mean percent difference for the corrected VDaw calculated from the Bohr equation was 5%. The present results suggest that the difference, although statistically significant, is small (0.19±0.24 ml/kg) and thus can be neglected under most conditions prevailing during mechanical ventilation. PaCO2 values were never over 51 mmHg (mean: 39.9±11.8 mmHg, range: 25–51 mmHg, except in one case: 63 mmHg in a 13.8-kg child), suggesting that rebreathing was not an important phenomenon in our patients. The other effects of insertion of the sensor were not measured in this study but Castle et al., studying accuracy of displayed values of VT in the pediatric ICU, by using CO2SMO Plus, demonstrated that this insertion had minimal effect on either VT or PIP, the average change being <1%, provided the appropriate sensor is used over a designated flow range [21]. However, these authors described some underestimations of recorded volumes at low flows with the pediatric pneumotachometer and did not recommend using this sensor in children with mean flows less than 4 l/min. This last study was not published when we started ours.
Like Fletcher et al. [3, 18], we found wide significant differences between VDaw and VDalv values by the two methods. The VDaw SBT-CO2/VDaw Bohr relation is affected by the slope of phase III. VDaw Bohr consists of VDaw plus part of VDalv. The magnitude of the alveolar part of VDaw Bohr is proportional to phase III. Thus, in children with normal slopes, Fletcher found that VDaw SBT-CO2 was 67% of VDaw Bohr. In children in whom phase III slope was increased, VDaw SBT-CO2 was only 51% of VDaw Bohr [3]. In contrast, Wenzel et al. found no significant difference between VDDaw and VDalv calculated by the two methods in 22 ventilated neonates [10]; nevertheless, in their study VDaw SBT-CO2 values were smaller than those by Bohr-Enghoff equations (VDaw SBT-CO2: 3.65±1.59 ml, VDaw Bohr: 5.27±2.44 ml). We observed no significant difference between VDphysiol calculated by the two methods, after correction was made for rebreathing of expired gas. Only when arterial-end tidal CO2 gradient is zero will the two methods give the same VDpysiol values. In our patients, the small gradient (0.81±3.4 mmHg) explains that VDphysiol values were quite similar with the two methods. VDalv cannot be estimated by using Bohr-Enghoff equations in contrast to SBT-CO2 analysis which enables VDalv calculation. However, determination of VDalv may be of importance because previous data suggest that quantification of the VDalv may be directly related to effective pulmonary perfusion [22] and that changes in phase III slope may reflect alveolar development and lung growth in infants [23].
Despite its pitfalls, volumetric capnography and VD/VT determination might be useful in many clinical situations: it could serve as a useful device to monitor adequacy of mechanical ventilation and help in evaluating ventilatory disturbances in patients with acute respiratory distress syndrome [2, 4, 5, 9, 11, 24]. Finally, VD/VT measurement could be used to evaluate the consistency of breathing before extubation [5].
Conclusion
Measurement of VDaw by the CO2SMO Plus was reproducible over a 1-h period, in ventilated children, provided ventilatory parameters were kept constant throughout the study. A more prolonged use will have to take into account the pitfalls of the method (damage by water condensate, alteration of end-tidal CO2 readings by deposition of secretions) [25, 26, 27, 28, 29]. Furthermore, to minimize the risk of hypercapnia, we recommend choosing the sensor with the minimal dead space, i.e., the neonatal one up to a mean flow of 5 l/min as suggested by Castle et al. [22].
References
Bouhuys A (1964) Respiratory dead space. In Fenn WO, Rahn H (Eds) Handbook of physiology. Washington American Physiological Society, pp 169–177
Arnold JH, Bower LK, Thompson JE (1995) Respiratory deadspace measurements in neonates with congenital diaphragmatic hernia. Crit Care Med 23:371–375
Fletcher R (1988) Invasive and non-invasive measurement of the respiratory deadspace in anesthetized children with cardiac disease. Anesth Analg 67:442–447
Arnold JH, Thompson JE, Benjamin PK (1993) Respiratory deadspace measurements in neonates during extracorporeal membrane oxygenation. Crit Care Med 21:1895–1900
Hubble CL, Gentile MA, Tripp DS, Craig DM, Meliones JM, Cheifetz IM (2000) Deadspace to tidal volume ratio predicts successful extubation in infants and children. Crit Care Med 28:2034–2040
Aitken RS, Clarke-Kennedy AE (1928) On the fluctuation in the composition of the alveolar air during the respiratory cycle in muscular exercise. J Physiol (London) 65:389–411
Fowler WS (1948) Lung function studies: II. The respiratory dead space. Am J Physiol 154:405–416
Fletcher R, Jonson B, Brew W (1981) The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth 53:77–88
Arnold JH, Thompson JE, Arnold LW (1996) Single breath CO2 analysis: description and validation of a method. Crit Care Med 24:96–102
Wenzel U, Wauer RR, Schmalish G (1999) Comparison of different methods for dead space measurements in ventilated newborns using CO2-volume plot. Intensive Care Med 25:705–713
Hsieh KS, Lee CL, Lin CC, Wu SN, Ko FY, Huang YF, Huang TC (2001) Quantitative analysis of end-tidal carbon dioxide during mechanical and spontaneous ventilation in infants and children. Pediatr Pulmonol 32:453–458
Bohr C (1891) Uber die Lungenatmung. Skand Archiv Physiol 2:236–268
Enghoff H (1938) Volumen ineficax Bemerkungen zur Frage des schadlichen Raumes. Uppsala Lakaref Furhand 44:191–218
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:59–174
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Koulouris NG, Latsi P, Dimitroulis J, Jordanoglou B, Gaga M, Jordanoglou J (2001) Noninvasive measurement of mean alveolar carbon dioxide tension and Bohr’s dead space during tidal breathing. Eur Respir J 17:1167–1174
Numa AH, Newth CJL (1996) Anatomic dead space in infants and children. J appl Physiol 80:1485–1489
Fletcher R (1984) Airway deadspace, end-tidal CO2 and Christian Bohr. Acta Anaesthesiol Scand 28:408–411
Marsh JM, Ingram D, Milner AD (1993) The effect of instrumental dead space on measurement of breathing pattern and pulmonary mechanics in the newborn. Pediatr Pulmonol 6:36–322
Fletcher R, Werner O, Nordstrom L, Jonson B (1983) Sources of error and their correction in the measurement of carbon dioxide elimination using the Siemens-Elema CO2 analyser. Br. J Anaesth 55:77
Castle RA, Dune CJ, Mok Q, Wade AM, Stocks J (2002) Accuracy of displayed values of tidal volume in the pediatric intensive care unit. Crit Care Med 30:2566–2574
Severinghaus JW, Stupfel M (1957) Alveolar dead space as an index of distribution of blood flow in pulmonary capillaries. J Appl Physiol 10:335–348
Ream RS, Schreiner MS, Neff JD (1995) Volumetric capnography in children: influence of growth on the alveolar plateau slope. Anesthesiology 82:64–73
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Jurban A, Tobin MJ (1996) Monitoring during mechanical ventilation. Clin Chest Med 17:453–473
Truwit JD, Rochester DF (1994) Monitoring the respiratory system of the mechanically ventilated patient. New Horizons 2:94–106
Schmitz BD, Shapiro BA (1995) Capnography. Respir Care Clin N Am 1:107–117
AARC (American Association for Respiratory Care) (1995) clinical practice guidelines. Capnography/capnometry during mechanical ventilation. Respir Care 40:1321–1324
Ahrens T, Wijeweera H, Ray S (1999) Capnography. A key underutilized technology. Crit Care Nurs North Am 11:49–62
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riou, Y., Leclerc, F., Neve, V. et al. Reproducibility of the respiratory dead space measurements in mechanically ventilated children using the CO2SMO monitor. Intensive Care Med 30, 1461–1467 (2004). https://doi.org/10.1007/s00134-004-2288-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2288-3