Abstract
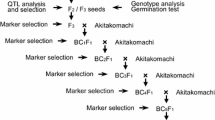
Low temperature is among the critical environmental factors that limit soybean production. To elucidate the genetic basis for chilling tolerance and identify useful markers, we conducted quantitative trait loci (QTL) analysis of seed-yielding ability at low temperature in soybean (Glycine max), using artificial climatic environments at usual and low temperatures and recombinant inbred lines derived from a cross between two contrasting cultivars in terms of chilling tolerance. We identified a QTL of a large effect (LOD > 15, r 2 > 0.3) associated with seed-yielding ability only at low temperature. The QTL was mapped near marker Sat_162 on linkage group A2, where no QTL for chilling tolerance has previously been identified. The tolerant genotype did not increase the pod number but maintained the seed number per pod and single seed weight, namely, the efficiency of seed development at low temperature. The effect of the QTL was confirmed in a segregating population of heterogeneous inbred families, which provided near-isogenic lines. The genomic region containing the QTL also influenced the node and pod numbers regardless of temperature condition, although this effect was not primarily associated with chilling tolerance. These results suggest the presence of a new major genetic factor that controls seed development specifically at low temperature. The findings will be useful for marker-assisted selection as well as for understanding of the mechanism underlying chilling tolerance in reproductive organs.




Similar content being viewed by others
References
Andaya VC, Mackill DJ (2003a) Map** of QTLs associated with cold tolerance during the vegetative stage in rice. J Exp Bot 54:2579–2585
Andaya VC, Mackill DJ (2003b) QTL conferring cold tolerance at the booting stage of rice using recombinant inbred lines from a japonica x indica cross. Theor Appl Genet 106:1084–1090
Awal MA, Ikeda T (2003) Controlling canopy formation, flowering, and yield in field-grown stands of peanut (Arachis hypogaea L.) with ambient and regulated soil temperature. Field Crop Res 81:121–132
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trend Plant Sci 12:444–451
Churchill G, Doerge R (1994) Empirical threshold values for quantitative trait map**. Genetics 138:963–971
Desclaux D, Huynh TT, Roumet P (2000) Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci 40:716–722
Fehr WE, Caviness CE, Burmood PT, Pennington J (1971) Stage of development description of soybeans, Glycine max (L.) Merrill. Crop Sci 11:929–931
Funatsuki H, Matsuba S, Kawaguchi K, Murakami T, Sato Y (2004) Methods for evaluation of soybean chilling tolerance at the reproductive stage under artificial climatic conditions. Plant Breed 123:558–563
Funatsuki H, Kawaguchi K, Matsuba S, Sato Y, Ishimoto M (2005) Map** of QTL associated with chilling tolerance during reproductive growth in soybean. Theor Appl Genet 111:851–861
Funatsuki H, Hajika M, Hagihara S, Yamada T, Tanaka Y, Tsuji H, Ishimoto M, Fu**o K (2008) Confirmation of the location and the effects of a major QTL controlling pod dehiscence, qPDH1, in soybean. Breed Sci 58:63–69
Holland JB (2007) Genetic architecture of complex traits in plants. Curr Opin Plant Biol 10:156–161
SAS Institute (1996) SAS/STAT user’s guide, vols 1 and 2, version 6, 4th edn. Cary, USA
Jompuk C, Fracheboud Y, Stamp P, Leipner J (2005) Map** of quantitative trait loci associated with chilling tolerance in maize (Zea mays L.) seedlings grown under field conditions. J Exp Bot 56:1153–1163
Karim A, Fukamachi H, Komori S, Ogawa K, Hidaka T (2003) Growth, yield and photosynthetic activity of Vigna radiata L. Grown at different temperature and light levels. Plant Prod Sci 6:43–49
Kuroki M, Saito K, Matsuba S, Yokogami N, Shimizu H, Ando I, Sato Y (2007) A quantitative trait locus for cold tolerance at the booting stage on rice chromosome 8. Theor Appl Genet 111:593–600
Kurosaki H, Yumoto S (2003) Effects of low temperature and shading during flowering on the yield components in soybeans. Plant Prod Sci 6:17–23
Kurosaki H, Yumoto S, Matsukawa I (2004) Correlation of cold-weather tolerance with pubescence color and flowering time in yellow hilum soybeans in Hokkaido. Breed Sci 54:303–311
Lander ES, Botstein D (1989) Map** Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Leipner J, Jompuk C, Camp KH, Stamp P, Fracheboud Y (2008) QTL studies reveal little relevance of chilling-related seedling traits for yield in maize. Theor Appl Genet 116:555–562
Leung H (2008) Stressed genomics—bringing relief to rice fields. Curr Opin Plant Biol 11:201–208
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Manly KF, Cudmore RH Jr, Meer JM (2001) Map Manager QTX, cross-platform software for genetic map**. Mamm Genome 12:930–932
Morrison MJ, Voldeng HD, Guillemette RJD (1994) Soybean pubescence color influences seed yield in cool-season climates. Agron J 86:796–799
Munoz-Perea CG, Teran H, Allen RG, Wright JL, Westermann DT, Singh SP (2006) Selection for drought resistance in dry bean landraces and cultivars. Crop Sci 46:2111–2120
Palmer RG, Pfeiffer TW, Buss GR, Kilen TC (2004) Qualitative genetics. In: Boerma HR, Specht JE (eds) Soybeans: improvement, production, and uses, 3rd edn. American Soc Agron Inc Crop Sci Soc America Inc Soil Sci Soc America Inc Publishers, Madison, pp 137–233
Penfield S (2008) Temperature perception and signal transduction in plants. New Phytol 179:615–628
Rainey KM, Griffiths PD (2005) Diallel analysis of yield components of snap beans exposed to two temperature stress environments. Euphytica 142:43–53
Raper CD Jr, Kramer PJ (1987) Stress physiology. In: Wilcox JR (ed) Soybeans: improvement, production, and uses, 2nd edn. American Soc Agronomy, Inc. Crop Sci Soc America. Inc. Soil Sci Soc America Inc. Publishers, Madison, pp 589–642
Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37:1141–1146
Saito K, Miura K, Nagano K, Hayano-Saito Y, Araki H, Kato A (2001) Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theor Appl Genet 103:862–868
Satake T, Hayase H (1970) Male sterility caused by cooling treatment at the young microspore stage in rice plants. V. Estimation of pollen developmental stage and the most sensitive stage to coolness. Proc Crop Sci Soc Jpn 39:468–473
Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao Z, Zheng CC (2007) Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176:70–81
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Shirai S, Yumoto S, Matsukawa I, Tanaka Y, Hagihara S, Kurosaki H, Sumida S, Yamazaki T, Suzuki C, Ohnishi S (2004) A new soybean variety “Toyoharuka” (in Japanese). http://www.affrc.go.jp/ja/research/seika/data_nics/h16/d16006
Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, Specht JE, Cregan PB (2004) A new integrated genetic linkage map of the soybean. Theor Appl Genet 109:122–128
Takahashi R, Asanuma S (1996) Association of t gene with chilling tolerance in soybean. Crop Sci 36:559–562
Takahashi R, Benitez ER, Funatsuki H, Ohnishi S (2005) Soybean maturity and pubescence color genes improve chilling tolerance at high latitude regions. Crop Sci 45:1387–1393
Takeuchi Y, Hayasaka H, Chiba B, Tanaka I, Shimano T, Yamagishi M, Nagano K, Sasaki T, Yano M (2001) Map** quantitative trait loci controlling cool-temperature tolerance at booting stage in temperate Japonica rice. Breed Sci 51:191–197
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Ann Rev Plant Physiol Plant Mol Biol 50:571–599
Tuinstra MR, Ejeta G, Goldsbrough PB (1997) Heterogeneous inbred family (HIF) analysis: a method for develo** near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet 95:1005–1011
Wang SCJ, Basten CJ, Zeng Z-B (2005) Windows QTL cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC, USA
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1a is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann Rev Plant Biol 57:781–803
Yamanaka N, Ninomiya S, Hoshi M, Tsubokura Y, Yano M, Nagamura Y, Sasaki T, Harada K (2001) An informative linkage map of soybean reveals QTL for flowering time, leaflet morphology and regions of segregation distortion. DNA Res 8:61–72
Yamanaka N, Watanabe S, Toda K, Hayashi M, Fuchigami H, Takahashi R, Harada K (2005) Fine map** of the FT1 locus for soybean flowering time using a residual heterozygous line derived from a recombinant inbred line. Theor Appl Genet 110:634–639
Yin X, Struik PC, Kropff MJ (2004) Role of crop physiology in predicting gene-to-phenotype relationships. Trend Plant Sci 9:426–432
Acknowledgments
The authors thank Dr. Y. Sato for his encouragement and support for this work. The technical assistance of R. Narita, S. Furuhata, K. Yoshida and R. Sugisawa is gratefully acknowledged. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 18580011) and by a grant from the National Agricultural Research Organization (Development of Innovative Crops through the Molecular Analysis of Useful Genes) to H. F.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. T. Nguyen.
Rights and permissions
About this article
Cite this article
Ikeda, T., Ohnishi, S., Senda, M. et al. A novel major quantitative trait locus controlling seed development at low temperature in soybean (Glycine max). Theor Appl Genet 118, 1477–1488 (2009). https://doi.org/10.1007/s00122-009-0996-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-0996-3