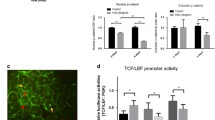
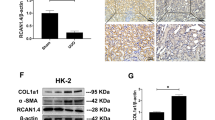
Abstract
Despite substantial progress being made in understanding the mechanisms contributing to the pathogenesis of renal fibrosis, there are only a few therapies available to treat or prevent renal fibrosis in clinical use today. Therefore, identifying the key cellular and molecular mediators involved in the pathogenesis of renal fibrosis will provide new therapeutic strategy for treating patients with chronic kidney disease (CKD). β-Arrestin-1, a member of β-arrestin family, not only is a negative adaptor of G protein-coupled receptors (GPCRs), but also acts as a scaffold protein and regulates a diverse array of cellular functions independent of GPCR activation. In this study, we identified for the first time that β-arrestin-1 was upregulated in the kidney from mice with unilateral ureteral obstruction nephropathy as well as in the paraffin-embedded sections of human kidneys from the patients with diabetic nephropathy, polycystic kidney, or uronephrosis, which normally causes renal fibrosis. Deficiency of β-arrestin-1 in mice significantly alleviated renal fibrosis by the regulation of inflammatory responses, kidney fibroblast activation, and epithelial-mesenchymal transition (EMT) in both in vivo and in vitro studies. Furthermore, we found that among the major isoforms of Wnts, Wnt1 was regulated by β-arrestin-1 and gene silencing of Wnt1 inhibited the activation of β-catenin and suppressed β-arrestin-1-mediated renal fibrosis. Collectively, our results indicate that β-arrestin-1 is one of the critical components of signal transduction pathways in the development of renal fibrosis. Modulation of these pathways may be an innovative therapeutic strategy for treating patients with renal fibrosis.
Key messages
-
β-Arrestin-1 was upregulated in the kidney from mice with UUO nephropathy.
-
β-Arrestin-1 regulated kidney fibroblast activation and epithelial-mesenchymal transition.
-
β-Arrestin-1 exacerbated renal fibrosis via mediating Wnt1/β-catenin signaling.







Similar content being viewed by others
References
Zeisberg M, Neilson EG (2010) Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21:1819–1834
Boor P, Ostendorf T, Floege J (2010) Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6:643–656
Pupo AS, Duarte DA, Lima V, Teixeira LB, Parreiras ESLT, Costa-Neto CM (2016) Recent updates on GPCR biased agonism. Pharmacol Res 112:49–57
Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC (2017) G protein-coupled receptor-G-protein beta gamma-subunit signaling mediates renal dysfunction and fibrosis in heart failure. J Am Soc Nephrol 28:197–208
Kang DS, Tian X, Benovic JL (2014) Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol 27:63–71
Spiegel A (2003) Cell Signaling. Beta-arrestin—not just for G protein-coupled receptors. Science 301:1338–1339
Kendall RT, Luttrell LM (2009) Diversity in arrestin function. Cell Mol Life Sci 66:2953–2973
Ma L, Pei G (2007) Beta-arrestin signaling and regulation of transcription. J Cell Sci 120:213–218
Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L (2006) Beta-arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103:14110–14115
Quack I, Woznowski M, Potthoff SA, Palmer R, Konigshausen E, Sivritas S, Schiffer M, Stegbauer J, Vonend O, Rump LC et al (2011) PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem 286:12959–12970
Buelli S, Rosano L, Gagliardini E, Corna D, Longaretti L, Pezzotta A, Perico L, Conti S, Rizzo P, Novelli R et al (2014) Beta-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol 25:523–533
Liu H, Yi F, Yang H (2016) Adaptive grou** cloud model shuffled frog lea** algorithm for solving continuous optimization problems. Comput Intell Neurosci 2016:5675349
Lovgren AK, Kovacs JJ, **e T, Potts EN, Li Y, Foster WM, Liang J, Meltzer EB, Jiang D, Lefkowitz RJ et al (2011) Beta-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med 3:74ra23
YJ G, Sun WY, Zhang S, JJ W, Wei W (2015) The emerging roles of beta-arrestins in fibrotic diseases. Acta Pharmacol Sin 36:1277–1287
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, **ao K et al (2011) A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 477:349–353
Wang HM, Dong JH, Li Q, Hu Q, Ning SL, Zheng W, Cui M, Chen TS, **e X, Sun JP et al (2014) A stress response pathway in mice upregulates somatostatin level and transcription in pancreatic delta cells through Gs and beta-arrestin 1. Diabetologia 57:1899–1910
Ai J, Nie J, He J, Guo Q, Li M, Lei Y, Liu Y, Zhou Z, Zhu F, Liang M et al (2015) GQ5 hinders renal fibrosis in obstructive nephropathy by selectively inhibiting TGF-beta-induced Smad3 phosphorylation. J Am Soc Nephrol 26:1827–1838
Du P, Fan B, Han H, Zhen J, Shang J, Wang X, Li X, Shi W, Tang W, Bao C et al (2013) NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int 84:265–276
Yan Y, Ma L, Zhou X, Ponnusamy M, Tang J, Zhuang MA, Tolbert E, Bayliss G, Bai J, Zhuang S (2016) Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int 89:68–81
Ding Y, Kim S, Lee SY, Koo JK, Wang Z, Choi ME (2014) Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol 25:2835–2846
Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang S, Du P, Zhang X, Yi F (2009) NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol Biochem 24:619–626
Yi F, Zhang AY, Janscha JL, Li PL, Zou AP (2004) Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int 66:1977–1987
Wang X, Liu J, Zhen J, Zhang C, Wan Q, Liu G, Wei X, Zhang Y, Wang Z, Han H et al (2014) Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int 86:712–725
Zhou M, Tang W, Fu Y, Xu X, Wang Z, Lu Y, Liu F, Yang X, Wei X, Zhang Y et al (2015) Progranulin protects against renal ischemia/reperfusion injury in mice. Kidney Int 87:918–929
Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ (2009) Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 17:443–458
Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG (1997) Beta-arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 81:1021–1026
Zhou X, Fukuda N, Matsuda H, Endo M, Wang X, Saito K, Ueno T, Matsumoto T, Matsumoto K, Soma M et al (2013) Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am J Phys Renal Phys 305:F957–F967
Duffield JS (2014) Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124:2299–2306
Kriz W, Kaissling B, Le Hir M (2011) Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 121:468–474
Freedman NJ, Shenoy SK (2017) Regulation of inflammation by beta-arrestins: not just receptor tales. Cell Signal. https://doi.org/10.1016/j.cellsig.2017.02.008
Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ (2000) Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol 1:227–233
Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ (2003) Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 112:566–574
Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149:1192–1205
Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y (2014) Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol 26(1):107–120
He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20:765–776
Liu J, Li QX, Wang XJ, Zhang C, Duan YQ, Wang ZY, Zhang Y, Yu X, Li NJ, Sun JP et al (2016) Beta-arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis 7:e2183
Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD (2016) Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27:781–790
Chen W, LA H, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE (2001) Beta-arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci U S A 98:14889–14894
Qian C, Liu F, Ye B, Zhang X, Liang Y, Yao J (2015) Notch4 promotes gastric cancer growth through activation of Wnt1/beta-catenin signaling. Mol Cell Biochem 401:165–174
Funding
This study was supported by the National Science Fund for Distinguished Young Scholars to Yi F (81525005) and the National Nature Science Foundation of China (91642204, 81470958, 81371317, 81600570, and 81400730).
Author information
Authors and Affiliations
Contributions
In this manuscript, Huiyan Xu conducted the experiment, interpreted the data and wrote the manuscript. Quanxin Li, Jiang Liu, Jiaqing Zhu, Liang Li conducted the experiment and analyzed the data. Wang ZY, Zhang Y, Yu Sun, **peng Sun and Rong Wang assisted with the conduction of the experiment. Yi F designed the experiment, wrote the manuscript and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
All mice had unrestricted access to food/water in accordance with Institutional Animal Care and Use Committee procedures of Shandong University. The investigation conforms to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Human renal biopsies were conducted in accordance with the principles of the Declaration of Helsinki and were approved by the Research Ethics Committee of Shandong University after informed consent was obtained from the patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, H., Li, Q., Liu, J. et al. β-Arrestin-1 deficiency ameliorates renal interstitial fibrosis by blocking Wnt1/β-catenin signaling in mice. J Mol Med 96, 97–109 (2018). https://doi.org/10.1007/s00109-017-1606-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1606-5