Abstract
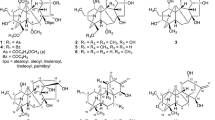
The comprehensive anti-cancer activities of cannabinoid acids and non-cannabinoids are not fully explored. Herein we report simple extraction, faster bioassay-guided fractionation, and HPLC-assisted purification of bioactive secondary metabolite and their identification. Anti-proliferative activity of six cannabinoid acids and eleven non-cannabinoids from the leaves of Cannabis sativa was evaluated. HPLC- assisted purification from the bioactive fractions leads to isolate one new compound Methoxy Canniprene (methoxy isoprenyl bibenzyl), including all six CBDA’s eleven non-cannabinoids in a single step, and NMR, HR-ESI-MS studies, and comparison with the literature data authenticated their structures. The crude extract (IC50 range: 18.0–37.2 μg/mL), Fraction A to D (11.9–222 μg/mL), and purified different cannabinoid and non cannabinoids (IC50 range: 0.5–100 μM) displayed potent anti-proliferative activities against almost all the tested five human cancer cell lines. Computational analysis based on molecular docking studies and absolute binding free energy calculation have identified targets for various cancer-associated proteins, including Aldehyde dehydrogenase 1 A1, Topoisomerase I, GSK-3 Beta, Protein Kinase C beta II, and Neurotrophic Tyrosine Kinase receptor type 1.











Similar content being viewed by others
Change history
22 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00044-024-03217-z
References
Andre CM, Hausman J-F, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7 .https://doi.org/10.3389/fpls.2016.00019.
Zou S, Kumar U. Cannabinoid receptors and the Endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833. https://doi.org/10.3390/ijms19030833.
National Academies of Sciences E, Medicine, Health, Medicine D, Board on Population H, Public Health P et al. The National Academies Collection: Reports funded by National Institutes of Health. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington (DC): National Academies Press (US).
Burstein SH. The cannabinoid acids: nonpsychoactive derivatives with therapeutic potential. Pharmacol Ther. 1999;82:87–96. https://doi.org/10.1016/S0163-7258(98)00069-2.
McPartland JM, MacDonald C, Young M, Grant PS, Furkert DP, Glass M. Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2017;2:87–95. https://doi.org/10.1089/can.2016.0032.
Lewis MM, Yang Y, Wasilewski E, Clarke HA, Kotra LP. Chemical profiling of medical cannabis extracts. ACS Omega. 2017;2:6091–103. https://doi.org/10.1021/acsomega.7b00996.
Martinenghi LD, Jønsson R, Lund T, Jenssen H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules. 2020;10. https://doi.org/10.3390/biom10060900.
Popp JR, Petrakis EA, Angelis A, Halabalaki M, Bonn GK, Stuppner H, et al. Rapid isolation of acidic cannabinoids from Cannabis sativa L. using pH-zone-refining centrifugal partition chromatography. J Chromatogr A. 2019;1599:196–202. https://doi.org/10.1016/j.chroma.2019.04.048.
Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–29. https://doi.org/10.1038/nrd4510.
Dadiotis E, Mitsis V, Melliou E, Magiatis P. Direct Quantitation of Phytocannabinoids by One-Dimensional 1H qNMR and Two-Dimensional 1H-1H COSY qNMR in Complex Natural Mixtures. Molecules. 2022;27:2965.
Aizikovich A. Anticancer Effect of New Cannabinoids Derived from Tetrahydrocannabinolic Acid on PANC-1 and AsPC-1 Human Pancreas Tumor Cells. J Pancreat Cancer. 2020;6:40–4. https://doi.org/10.1089/pancan.2020.0003.
Suzuki M, Takeda S, Okazaki H, Watanabe K, Takiguchi M, Aramaki H. Cannabidiolic Acid-Mediated Interference with AP-1 Transcriptional Activity in MDA-MB-231 Breast Cancer Cells. Nat Prod Commun. 2017;12:759–61.
Nahler G. Cannabidiol and other phytocannabinoids as cancer therapeutics. Pharmaceut Med. 2022;36:99–129. https://doi.org/10.1007/s40290-022-00420-4.
Sainz-Cort A, Müller-Sánchez C, Espel E. Anti-proliferative and cytotoxic effect of cannabidiol on human cancer cell lines in presence of serum. BMC Res Notes. 2020;13:389. https://doi.org/10.1186/s13104-020-05229-5.
Durante C, Anceschi L, Brighenti V, Caroli C, Afezolli C, Marchetti A, et al. Application of experimental design in HPLC method optimisation for the simultaneous determination of multiple bioactive cannabinoids. J Pharm Biomed Anal. 2022;221:115037. https://doi.org/10.1016/j.jpba.2022.115037.
Janatová A, Doskočil I, Božik M, Fraňková A, Tlustoš P, Klouček P. The chemical composition of ethanolic extracts from six genotypes of medical cannabis (Cannabis sativa L.) and their selective cytotoxic activity. Chem Interact. 2022;353:109800.
Shor DB-A, Hochman I, Gluck N, Shibolet O, Scapa E. The Cytotoxic Effect of Isolated Cannabinoid Extracts on Polypoid Colorectal Tissue. Int J Mol Sci. 2022;23:11366.
Tomko AM, Whynot EG, Ellis LD, Dupré DJ. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers. 2020;12:1985.
Helcman M, Šmejkal K. Biological activity of Cannabis compounds: A modern approach to the therapy of multiple diseases. Phytochem Rev. 2022;21:429–70.
Mingaleeva RN, Solovieva VV, Blatt NL, Rizvanov AA. Application of cell and tissue cultures for potential anti-cancer/oncology drugs screening in vitro. Cell. Transpl. Tissue Eng. 2013;8:20–8.
Open Targets Platform. https://platform.opentargets.org/ (accessed 2023).
Open Targets: A Platform for Therapeutic Target Identification and Validation.
Bank PD. Protein Data Bank. Nat New Biol. 1971;233:223.
RCSB PDB: Homepage. https://www.rcsb.org/ (accessed 2023).
Trott O, Olson AJ. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J Comput Chem. 2010;31:455–61.
Eswar N, Eramian D, Webb B, Shen M-Y, Sali A. Protein Structure Modeling with MODELLER. In Structural Proteomics; Springer, 2008; pp 145–59.
DE Shaw Research. Desmond Molecular Dynamics System. 2016.
DeLano WL. The PyMOL Molecular Graphics System. 2002.
Jiménez J, Škalič M, Martínez-Rosell G, De Fabritiis G. K DEEP: Protein–Ligand Absolute Binding Affinity Prediction via 3D-Convolutional Neural Networks. J Chem Inf Model. 2018;58:287–96. https://doi.org/10.1021/acs.jcim.7b00650.
Acknowledgements
Yedukondalu Nalli acknowledges fellowship (Aroma Mission project phase-II) from Council of Scientific and Industrial Research (CSIR). Authors are thankful to Director, CSIR-IIIM, Jammu for providing necessary facility and support during the study.
Author contributions
MKV conceived the study. YKN, RS, and JPB performed the extraction and isolation of compounds by using column chromatography and preparative HPLC. SB and TA performed the biological experiments. MKV and YKN carried out structure elucidation of the isolated compounds and MKV drafted the manuscript. AG supervises the work of biological activity and compiled the data. SSB and YPB carried out in silico study and interpretation of the data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nalli, Y., Bharti, S., Amin, T. et al. Bioassay-guided fractionations of Cannabis sativa extract and HPLC-assisted purifications of anti-proliferative active fractions lead to the isolation of 16 known and one new phytomolecule and their in-silico analysis. Med Chem Res 33, 635–650 (2024). https://doi.org/10.1007/s00044-024-03199-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-024-03199-y