Abstract
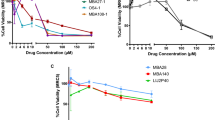
The search for an effective small molecule against the severe acute respiratory syndrome related coronavirus 2 (SARS-CoV-2) is a challenge that remains even after the end of the Coronavirus disease 2019 (COVID-19) global health emergency. The indole-based ferulic acid derivatives were synthesized in this work and evaluated for their in vitro cytotoxic profiles and anti-SARS-CoV-2 activity. Compounds 1 and 2 decreased the number of genomic copies of SARS-CoV-2 in a dose-dependent manner, with IC50 values of 70.85 µM and 68.28 µM, respectively, with no significant cytotoxicity up to 100 µM against uninfected Vero cells. In order to search for a possible molecular target of these compounds, their activity against the two SARS-CoV-2 cysteine proteases, Mpro and PLpro, in addition to the human cysteine protease cathepsin L (hCatL) was investigated. However, they did not display significant activity against any of these proteases and, therefore, their mechanism of action remains unclear. Our findings suggest that the activity may be related to antioxidant properties of 1 and 2, since the presence of a phenolic group is critical for the antiviral activity.







Similar content being viewed by others
References
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–54. https://doi.org/10.1038/s41579-020-00459-7
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–97. https://doi.org/10.1016/S0140-6736(20)30260-9
Adil MT, Rahman R, Whitelaw D, Jain V, Al-Taan O, Rashid F. et al. SARS-CoV-2 and the pandemic of COVID-19. Postgrad Med J. 2021;97:110–6. https://doi.org/10.1136/postgradmedj-2020-138386.
Huang Y, Yang C, Xu X, Xu W, Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–9. https://doi.org/10.1038/s41401-020-0485-4.
Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L. et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–12. https://doi.org/10.1126/science.abb3405.
Rut W, Lv Z, Zmudzinski M, Patchett S, Nayak D, Snipas SJ. et al. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design. Sci Adv. 2020;6:eabd4596. https://doi.org/10.1126/sciadv.abd4596.
Hillen HS, Kokic G, Farnung L, Dienemann C, Tegunov D, Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020;584:154–6. https://doi.org/10.1038/s41586-020-2368-8
Ashhurst AS, Tang AH, Fajtová P, Yoon MC, Aggarwal A, Bedding MJ. et al. Potent Anti-SARS-CoV-2 Activity by the Natural Product Gallinamide A and Analogues via Inhibition of Cathepsin L. J Med Chem. 2022;65:2956–70. https://doi.org/10.1021/acs.jmedchem.1c01494.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–.E8. https://doi.org/10.1016/j.cell.2020.02.052.
Liu T, Luo S, Libby P, Shi GP. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol Ther. 2020;213:107587. https://doi.org/10.1016/j.pharmthera.2020.107587
Yan R, Zhang Y, Li Y, **a L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–8. https://doi.org/10.1126/science.abb2762
Huang J, Song W, Huang H, Sun Q. Pharmacological Therapeutics Targeting RNA-Dependent RNA Polymerase, Proteinase and Spike Protein: From Mechanistic Studies to Clinical Trials for COVID-19. J Clin Med. 2020;9:1131. https://doi.org/10.3390/jcm9041131
Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A. et al. Cell Entry Mechanisms of SARS-CoV-2. Proc Natl Acad USA. 2020;117:11727–34. https://doi.org/10.1073/pnas.2003138117.
Narayanan A, Narwal M, Majowicz SA, Varricchio C, Toner SA, Ballatore C, et al. Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Comm Biol. 2022;5. https://doi.org/10.1038/s42003-022-03090-9.
Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nature Rev Microbiol. 2021;19:685–700. https://doi.org/10.1038/s41579-021-00630-8
Bhowmik D, Nandi R, Jagadeesan R, Kumar N, Prakash A, Kumar D. Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infect Genet Evol. 2020;84:104451 https://doi.org/10.1016/j.meegid.2020.104451
Antonopoulou I, Sapountzaki E, Rova U, Christakopoulos P. Ferulic Acid From Plant Biomass: A Phytochemical With Promising Antiviral Properties. Front Nutr. 2022;8:777576 https://doi.org/10.3389/fnut.2021.777576
Sgarbossa A, Giacomazza D, di Carlo M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients. 2015;7:5764–82. https://doi.org/10.3390/nu7075246
Liu Y, Shi L, Qiu W, Shi Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol Cell Toxicol. 2022;18:509–19. https://doi.org/10.1007/s13273-021-00219-5
Yin ZN, Wu WJ, Sun CZ, Liu HF, Chen WB, Zhan QP. et al. Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus niger. Biomed Environ Sci. 2019;32:11–21. https://doi.org/10.3967/bes2019.002.
Pernin A, Bosc V, Maillard MN, Dubois-Brissonnet F. Ferulic Acid and Eugenol Have Different Abilities to Maintain Their Inhibitory Activity Against Listeria monocytogenes in Emulsified Systems. Front Microbiol. 2019;10:137 https://doi.org/10.3389/fmicb.2019.00137
Gan X, Wang Z, Hu D. Synthesis of Novel Antiviral Ferulic Acid-Eugenol and Isoeugenol Hybrids Using Various Link Reactions. J Agric Food Chem. 2021;69:13724–33. https://doi.org/10.1021/acs.jafc.1c05521
Stompor-Gorący M, Machaczka M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. J Mol Sci. 2021;22:12889 https://doi.org/10.3390/ijms222312889
El Gizawy HA, Boshra SA, Mostafa A, Mahmoud SH, Ismail MI, Alsfouk AA. et al. Pimenta dioica (L.) Merr. Bioactive Constituents Exert Anti-SARS-CoV-2 and Anti-Inflammatory Activities: Molecular Docking and Dynamics, In Vitro, and In Vivo Studies. Molecules. 2021;26:5844 https://doi.org/10.3390/molecules26195844.
Pasquereau S, Galais M, Bellefroid M, Angona IP, Morot-Bizot S, Ismaili L. et al. Ferulic acid derivatives block coronaviruses HCoV-229E and SARS-CoV-2 replication in vitro. Sci Rep. 2022;12:20309. https://doi.org/10.1038/s41598-022-24682-9.
Cascioferro S, Petri GL, Parrino B, Hassouni BE, Carbone D, Arizza V. et al. 3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma. Molecules. 2020;25:329. https://doi.org/10.3390/molecules25020329.
Cascioferro S, Attanzio A, Di Sarno V, Musella S, Tesoriere L, Cirrincione G. et al. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Mar Drugs. 2019;17:35. https://doi.org/10.3390/md17010035.
Carbone A, Parrino B, Cusimano M, Spanó V, Montalbano A, Barraja P. et al. New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation. Mar Drugs. 2018;16:274. https://doi.org/10.3390/md16080274.
Dhuguru J, Skouta R. Role of Indole Scaffolds as Pharmacophores in the Development of Anti-Lung Cancer Agents. Molecules. 2020;25:1615. https://doi.org/10.3390/molecules25071615
Almaraz-Girón MA, Calderón-Jaimes E, Carrillo AS, Díaz-Cervantes E, Alonso EC, Islas-Jácome A. et al. Search for Non-Protein Protease Inhibitors Constituted with an Indole and Acetylene Core. Molecules. 2021;26:3817. https://doi.org/10.3390/molecules26133817.
Wang X, Sacramento CQ, Jockusch S, Chaves OA, Tao C, Fintelman-Rodrigues N. et al. Combination of antiviral drugs inhibits SARS-CoV2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture. Commun Biol. 2022;5:154 https://doi.org/10.1038/s42003-022-03101-9.
Zadeh NM, Asl NSM, Forouharnejad K, Ghadimi K, Parsa S, Mohammadi S, et al. Mechanism and adverse effects of COVID-19 drugs: a basic review. Int J Physiol Pathophysiol Pharmacol. 2021;13:102–9.
De Flora S, Balansky R, Maestra SL. Antioxidants and COVID-19. J Prev Med Hyg. 2021;62:1s3. https://doi.org/10.15167/2421-4248/jpmh2021.62.1S3.1895
Rehman MF, Akhter S, Batool AI, Selamoglu Z, Sevindik M, Eman R. et al. Effectiveness of Natural Antioxidants against SARS-CoV-2? Insights from the In-Silico World. Antibiotics. 2021;10:1011 https://doi.org/10.3390/antibiotics10081011.
Liu Y, Liang C, **n L, Ren X, Tian L, Ju X. et al. The development of Coronavirus 3C-Like protease (3CLpro) inhibitors from 2010 to 2020. Eur J Med Chem. 2020;206:112711 https://doi.org/10.1016/j.ejmech.2020.112711.
Vankadara S, Wong YX, Liu B, See YY, Tan LH, Tan QW. et al. A Head-to-Head Comparison of the Inhibitory Activities of 15 Peptidomimetic SARS-CoV-2 3CLpro Inhibitors. Bioorg Med Chem Lett. 2021;48:128263 https://doi.org/10.1016/j.bmcl.2021.128263.
Dai W, Jochmans D, **e H, Yang H, Li J, Su H. et al. Design, Synthesis, and Biological Evaluation of Peptidomimetic Aldehydes as Broad-Spectrum Inhibitors against Enterovirus and SARS-CoV-2. J Med Chem. 2022;65:2794–808. https://doi.org/10.1021/acs.jmedchem.0c02258.
Azevedo L, Serafim MSM, Maltarollo VG, Grabrucker AM, Granato D. Atherosclerosis Fate in the Era of Tailored Functional Foods: Evidence-based Guidelines Elicited from Structure-and Ligand-Based Approaches. Trends Food Sci Technol. 2022;128:75–89. https://doi.org/10.1016/j.tifs.2022.07.010
Serafim MS, Lavorato SN, Kronenberger T, Sousa YV, Oliveira GP, dos Santos SG. et al. Antibacterial activity of synthetic 1,3-bis(aryloxy)propan-2-amines against Gram-positive bacteria. Microbiologyopen. 2019;8:e814 https://doi.org/10.1002/mbo3.814.
Ashhurst AS, Tang AH, Fajtová P, Yoon MC, Aggarwal A, Bedding MJ. et al. Potent Anti-SARS-CoV-2 Activity by the Natural Product Gallinamide A and Analogues via Inhibition of Cathepsin L. J Med Chem. 2022;65:2956–70. https://doi.org/10.1021/acs.jmedchem.1c01494.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–80.E8. https://doi.org/10.1016/j.cell.2020.02.052.
Koenig SG, Dankwardt JW, Liu Y, Zhao H, Singh SP. A ligand-free, copper-catalyzed cascade sequence to indole-2-carboxylic esters. Tetrahedron Lett. 2010;51:6549–51. https://doi.org/10.1016/j.tetlet.2010.10.035
Tsotinis A, Afroudakis PA, Davidson K, Prashar A, Sugden D. Design, synthesis, and melatoninergic activity of new azido- and isothiocyanato-substituted indoles. J Med Chem. 2007;50:6436–40. https://doi.org/10.1021/jm7010723
Boraei ATA, El Ashry ESH, Barakat A, Ghabbour HA. Synthesis of New Functionalized Indoles Based on Ethyl Indol-2-carboxylate. Molecules. 2016;21:333 https://doi.org/10.3390/molecules21030333
Kaye M, Druce J, Tran T, Kostecki R, Chibo D, Morris J. et al. SARS-associated coronavirus replication in cell lines. Emerg Infect Dis. 2006;12:1 https://doi.org/10.3201/eid1201.050496.
Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S. et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. https://doi.org/10.1016/S1473-3099(20)30200-0.
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045.
Mellott DM, Tseng CT, Drelich A, Fajtová P, Chenna BC, Kostomiris DH. et al. A Clinical-Stage Cysteine Protease Inhibitor blocks SARS-CoV-2 Infection of Human and Monkey Cells. ACS Chem Biol. 2021;16:642–50. https://doi.org/10.1021/acschembio.0c00875.
Pillaiyar T, Flury P, Krüger N, Su H, Schäkel L, Da Silva EB. et al. Small-Molecule Thioesters as SARS-CoV-2 Main Protease Inhibitors: Enzyme Inhibition, Structure-Activity Relationships, Antiviral Activity, and X-ray Structure Determination. J Med Chem. 2022;65:9376–95. https://doi.org/10.1021/acs.jmedchem.2c00636.
Santos LH, Kronenberger T, Almeida RG, Silva EB, Rocha REO, Oliveira JC. et al. Structure-based identification of naphthoquinones and derivatives as novel inhibitors of main protease Mpro and papain-like protease PLpro of SARS-CoV-2. J Chem Inf Model. 2022;62:6553–73. https://doi.org/10.1021/acs.jcim.2c00693.
Ashhurst AS, Tang AH, Fajtová P, Yoon MC, Aggarwal A, Bedding MJ. et al. Potent Anti-SARS-CoV-2 Activity by the Natural Product Gallinamide A and Analogues via Inhibition of Cathepsin L. J Med Chem. 2022;65:2956–70. https://doi.org/10.1021/acs.jmedchem.1c01494.
Gaulton A, Herse A, Nowotka M, Bento P, Chambers J, Mendez D. et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017;45:D945–D954. https://doi.org/10.1093/nar/gkw1074.
Landrum G. Rdkit documentation. Release. 2013;1:4.
Cereto-Massagué A, Ojeda MJ, Valls C, Mulero M, Garcia-Vallvé S, Pujadas G. Molecular fingerprint similarity search in virtual screening. Methods. 2015;71:58–63. https://doi.org/10.1016/j.ymeth.2014.08.005
Berthold MR, Cebron N, Dill F, Gabriel TR, Kötter T, Meinl T, et al. Wiswedel. KNIME-the Konstanz information miner: version 2.0 and beyond. AcM SIGKDD explorations. Newsletter. 2009;11:26–31.
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support (CAPES grant numbers 88887.595578/2020-00 and 88887.684031/2022-00 for M.S.M.S fellowship). JGCdR received CNPq grant (No 407779/2021-3). SARS-CoV-2 was isolated from a patient with COVID-19 in São Paulo, Brazil, and was kindly provided by Dr. Edison Durigon, from the Departamento de Microbiologia of Instituto de Ciências Biomédicas of Universidade de São Paulo (USP), Brazil. This virus was obtained by REDE VIRUS (MCTI/Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verzola, M.M.S.A., de Almeida Marques, D.P., da Silva, E.B. et al. Synthesis of indole-based ferulic acid derivatives and in vitro evaluation of antiviral activity against SARS-CoV-2. Med Chem Res 32, 2256–2267 (2023). https://doi.org/10.1007/s00044-023-03134-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03134-7