Summary
The bioavailability of two oral formulations of trimipramine, tablets and solution, was performed in twelve healthy volunteers, in a cross-over study. Each formulation was administered in the morning after a fasted period, and in the evening after a meal, in order to evaluate the role of both administration time and food consumption on the plasma kinetic parameters, under usual therapeutic conditions. A high interindividual variability of data was found.
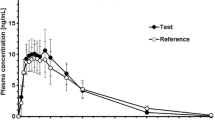
First, the extent of bioavailability was identical for the two formulations but the rate of bioavailability seemed to be different, with the p.o. solution, being more rapidly absorbed (tmax=1.50 h).
The effect of administration time was more obvious for the solution as shown by a lower quantitative absorption as well as a delay in time to reach the maximal concentration.
Regardless of formulation and administration time, the ty1/2 β was about 10 hours and the mean MRT value was 11 hours.
Similar content being viewed by others
References
Kristof F.E., Lehmann H.E., Ban T.A. (1967): Systematic studies with trimipramine, a new antidepressive drug. Can. Psychiatr. Ass. J., 12, 517.
Vauterin C., Bazot M. (1979): A double-blind controlled trial of amineptine versus trimipramine in depression. Curr. Med. Res. Opin., 6, 101–106.
Caille G., Besner J.G., Laçasse Y., Vezina M. (1980): Pharmacokinetic characteristics of two different formulations of trimipramine determined with a new GLC method. Biopharm. Drug Dispos., 1, 187–194.
Abernethy D.R., Greenblatt D.J., Shader R.I. (1984): Trimipramine kinetics and absolute bioavailability. Use of gas-liquid chromatography with nitrogen-phosphorus detection. Clin. Pharmacol. Ther., 35, 348–353.
Cajlle G. et al. (1984): Pharmacokinetic and clinical parameters of zopiclone and trimipramine when administered simultaneously to volunteers. Biopharm. Drug Dispos., 5, 117–125.
Bougerolle A.M., Chabard J.L., Jbilou M., et al. (1988): Simultaneous determination of trimipramine and its demethylated metabolites in plasma by gas chromatography-mass spectrometry. J. Chromatogr., 434, 232–238.
Gibaldi M., Perrier D. (1982): Pharmacokinetics, ed. 2, New-York, Marcel Dekker.
Taburet A.M., Steimer J.L., Doucet D., Singlas E. (1986): Le temps de présence moyen dans l’organisme: un nouveau paramètre pharmacocinétique? Thérapie, 41, 1–10.
Dunnett C.W. (1955): Am. Stat Ass. J., 1096–1121.
Westlake W.J. (1972): Use of confidence intervals in analysis of comparative bioavailability trials. J. Pharm. Sci., 61, 1340–1341.
Hollander M., Wolfe D.A. (1973): Non parametric statistical methods. New York, Wiley, Ch. 4.
Snedelor G.W. (1962): Statistical methods. The Iowa State University Press, Ames.
Riegelmann S., Collier P. (1980): The applications of statistical moment theory in the evaluation of in-vivo dissolution time and absorption time. J. Pharmacokinet. Biopharm., 8, 509–534.
Haginaka J., Yamaoka K., Nakagawa T. et al. (1979): Evaluation of effect of food ingestion on bioavailability of cephalexin by moment analysis. Chem. Pharm. Bull., 27, 3156–3159.
Bougerolle A.M., Chabard J.X., Dordain G., Gaillot J., Piron J.J., Berger J.A. (1984): Compared bioavailability of three oral forms of metapramine in human volunteers. Thérapie, 39, 619–624.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bougerolle, A.M., Chabard, J.L., Jbilou, M. et al. Chronopharmacokinetic and bioequivalence studies of two formulations of trimipramine after oral administration in man. Eur. J. Drug Metab. Pharmacokinet. 14, 139–144 (1989). https://doi.org/10.1007/BF03190854
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190854