Abstract
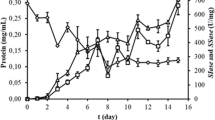
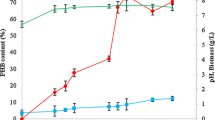
Optimal substrate feeding strategy in bioreactor operation was investigated to increase the production of secondary metabolite in a high density culture of plant cell. It was accomplished by the previously proposed structured kinetic model that describes the cell growth and synthesis of the secondary metabolite, berberine, in a batch suspension culture ofThalictrum rugosum. Four types of operation strategies for sugar feeding intoT. rugosum culture were proposed based on the model, which were the periodic fedbatch operations to maintain the cell activity, the cell viability, and the specific production rate, and the perfusion operation to maintain the specific production rate. From the simulation results of these strategies, it could be found that the periodic fed-batch operation and the perfusion operation could achieve the higher volumetric production of berberine (mg berberine/L) and specific production yield (mg berberine/g dry cell weight) than those of batch cultures. Although the highest productivity (mg berberine/day) of berberine could be achieved by the periodic fed-batch operation to maintain the cell activity compared with the other strategies in the periodic fed-batch operations, the specific production yield was low due to the higher maximum dry cell weight than other cases. The periodic fed-batch operation to maintain cell viability resulted in the highest volumetric production of berberine and specific production yield compared with the other strategies. In the cases of maintaining the specific production rate, the per-formance of the periodic fed-batch operation was better than that of the perfusion operation in the respect of the volumetric production and productivity of berberine. In order to increase the volumetric production of berberine and to get the highest specific production yield, the periodic fed-batch operation to maintain cell viability could be chosen as the optimal operating strategy in high density, culture ofT. rugosum plant cell.
Similar content being viewed by others
Abbreviations
- A :
-
activity (g/g)
- FI :
-
relative fluorescence intensity
- k :
-
rate constant (day−1)
- K :
-
Monod constant (g/L)
- P :
-
product concentration (g/L)
- S :
-
substrate concentration (g/L)
- t :
-
time (days)
- V :
-
viability (g/g)
- X :
-
biomass concentration (g/L)
- Y :
-
yield coefficient (g/g)
- α:
-
Growth-associated production constant (gg−1 day−1)
- β:
-
nongrowth-associated production constant (gg−1 day−1)
- ϰ:
-
cell expansion coefficient (day−1)
- μ:
-
specific growth rate (day−1)
- λ:
-
product degradation constant (gg−1 day−1)
- γ:
-
product release coefficient by cell lysis (g/g)
- θ:
-
function for cell expansion
- ϕ:
-
function for activity loss
- c:
-
conversion from sucrose
- d:
-
dry weight or death
- E:
-
extracellular
- f:
-
fresh weight
- F:
-
fructose
- G:
-
glucose
- I:
-
intracellular
- L:
-
lag phase
- m:
-
maximum
- o:
-
initial
- S:
-
sucrose
- t:
-
total
- ad:
-
active-viable cell
- dd:
-
dead cell
- nd:
-
nonactive-viable cell
- vd:
-
viable cell
- P/SF :
-
product from fructose
- P/SG :
-
product from glucose
- X/SF :
-
biomass from fructose
- X/SG :
-
biomass from glucose
References
Prenosil, J. E. and H. Pedersen (1983) Immobilized plant cell reactors.Enzyme Microb. Technol. 5: 323–331.
Panda, A. K., S. Mishira, V. S. Bisaria, and S. S. Bhojwani, (1989) Plant cell reactors-a perspective.Enzyme Microb. Technol. 11: 386–397.
Buitelaar, R. M. and J. M. Tramper (1992) Strategies to improve the production of secondary metabolites with plant cell culture; a literature review.J. Biotechnol. 23: 111–141.
Kim, D. I., H. Pedersen, and C. K. Chin (1991) Development of process strategies for berberine production in plant cell suspension cultures.J. Biotechnol. 21: 201–208.
Glicklis, R., D. Mills, D. Sitton, W. Stortelder, and J. C. Merchuk (1998) Polysaccharide production by plant cells in suspension: experiments and mathematical modeling.Biotechnol. Bioeng. 57: 732–740.
Takeda, T., Y. Takeuchi, M. Seki, S. Furusaki, and H. Matsuoka (1998) Kinetic analysis of cell growth and vitamin E production in plant cell culture ofCarthamus tinctorius using a structured model.Biochemical Eng. J. 1: 233–242.
Choi, J. W., Y. K. Kim, W. H. Lee, H. Pedersen, and C. K. Chin (1999) Kinetics of cell growth and secondary metabolites synthesis in, plant cell culture ofThalictrum rugosum using a structured model.Biotechnol. Bioprocess Eng. Submitted.
Westage, P. J., W. R. Curtis, A. H. Emergy, P. M. Hasegawa, and P. F. Heinstein (1991) Approximation of continuous growth ofCephalotaxus harringtonia plant cell cultures using fed-batch operation.Biotechnol. Bioeng. 38: 241–248.
Su, W. W. and A. E. Humphrey (1992) p. 266 In: S. Furusaki, I. Endo, and R. Matsuno (ed)Biochem. Eng. for 2001. Springer-Verlag Press. Tokyo, Japan.
Metzler, C. M., G. L. Elfring, and A. J. McEwen (1974) A package of computer programs for pharmacokinetic modelling.Biometrics 30: 562–563.
Seinfeld, J. and L. Lapidus (1970)Process Modeling, Estimation and Identification. Prentice Hall Inc. Englewood Cliffs, NJ, U.S.A.
Matsubara K., S. Kitani, T. Yoshioka, T. Morimoto, Y. Fujita, and Y. Yamada (1989) High density culture ofCoptis japonica cells increases berberine production.J. Chem. Technol. Biotechnol. 46: 61–69.
Tanaka, H. (1987) Large-scale cultivation of plant cells at high density: a review.Process Biochem. 22: 106–113.
Jolicoeur, M., C. Chavarie, P. Carreau, and J. Archambault (1992) Development of a helicalribbon-impeller bioreactor for high-density plant cell suspension culture.Biotechnol. Bioeng. 39: 511–521.
Pepin, M., J. Archambault, C. Chavarie, and F. Cormier (1995) Growth kinetics ofVitas vinifera cell suspension cultures: I. shake flask cultures.Biotechnol. Bioeng. 47: 131–138.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, JW., Kim, YK., Lee, W.H. et al. Bioreactor operating strategy inThalictrum rugosum plant cell culture for the production of berberine. Biotechnol. Bioprocess Eng. 4, 138–146 (1999). https://doi.org/10.1007/BF02932384
Issue Date:
DOI: https://doi.org/10.1007/BF02932384