Summary
The effects of 3, 4-DHAP on hypoxic vasoconstriction response in pulmonary (PA) and basilar arterial (BA) rings of rabbits and their mechanism were compared in vitro.
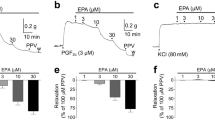
3,4-DHAP in different concentration (2. 64 × 10-4,7. 92 × 10-4,2. 376 × 10-3 mol/L) decreased the basal tone of PA rings by 32. 39 ± 9. 4 mg, 68. 96± 26. 54 mg and 145. 60 ± 58. 07 mg respectively, while the tension of the BA rings was decreased by 13. 80 ±5. 08 mg, 17.18 ± 3. 36 mg and 25. 00 ± 4. 02 mg respectively. In PA rings it also decreased the percentage increase in tension induced by hypoxia (TIH%) from the control value 48.82 ± 5.75% to 10.02 ± 3.62%, 2. 14 ± 0.96%, and 0. 00 % respectively, while in BA rings from 27. 27 ± 5.78% to 11. 23 ± 2.71%, 7.49 ± 1.62%, and 1.45 ± 1.13% respectively. The effects of 3, 4-DHAP on TIH% were partially blocked by indomethacin 10 M and L-NAME 10 M.
The results showed that 3, 4-DHAP can decrease the hypoxic pulmonary and basilar vasoconstriction in vitro, which can be partially inhibited by cyclooxygenase inhibitor and NO/EDRF inhibitor.
Similar content being viewed by others
References
Archer S L, Tolins J P, Weir E K. Hypoxic pulmonary vasoconstriction is enhanced by inhibition of synthesis of the endothelium derived relaxing factor. Biochem Biophys Res Commun, 1987; 164: 1198
1977; 8: 7
Brown I P, Thompson C I, and Belloni F L. Role of nitric oxide in hypoxic coronary vasodilation in isolated perfused guinea pig heart. Am JPhysiol, 1993t 264: H821
Cocks T M, Angus J A. Endothelium dependent relaxation of coronary arteries by NE and Serotonin. Nature, 1983; 305: 627.
3,4-1981; 2(2);107–110.
Gleason C A, Hamm C, Jones M Det al. Effect of acute hypoxaemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am JPhysiol, 1990; 258: H1064.
Haught W H, Yang B C, Lawson D Let al. Slow channel calcium blocker attenuates reoxygenation mediated vascular contraction. J Cardiovasc Pharmacol, 1992; 19: 701
Holden W E, McCall E. Hypoxia induced contraction of porcine pulmonary artery strips depend upon intact endothelium. Exp Lung Res, 1984; 7: 101
1993; 28: 333
** N, Packer C S, Rhoades R A. Pulmonary arterial hypoxic signal transduction. Am J Physiol, 1992; 263: L73
** X R, Fan M, Wang Z Qet al. Strain differences in pulmonary vascular responsiveness to hypoxia in rats. J Tongji Med Univ, 1990; 10: 134
Usha J. Rajand Prscilla C. Role of eicosanoids in hypoxic vasoconstriction in isolated Lamb lungs. Am J Physiol, 1987; 251: H626
Katusic Z S, Vanhoutte P M. Anoxic contraction in canine cerebral contraction in isolated canine cerebral arteries; contribution of endothelium derived factors, metabolites of arachidonic acid, and calcium entry. J Cardiovasc Pharmacol, 1986; 8, (Suppl 8): S97
Kovitz K L, Alesko T D W, Sylvester J Tet al. Endothelium derived relaxing and contracting factors contribute to hypoxic response of PA. Am J Physiol, 1993; 265: H1139
1993; 22: 91
Martin D, Barnes S D, Wetzel R D. Acute hypoxia alters eicosanoids production of perfused PA endothelial cells in culture. Prostaglandins, 1992; 43: 371
MC Murtry, I F, Davidson A B, Reeves J Tet al. Inhibition of hypoxic pulmonary vasoconstriction on calcium antagonists in isolated rats lungs. Circ Res, 1976; 38: 99
Nuramatsu M, Shimizu L, Assano H.et al. Hypoxia elicited contraction of aorta and coronary artery via removal of EDRF. Am J Physiol, 1992; 263: H1339
Tseng C M, Goodman L W, Rubin L Jet al. N G. monomethyl-L-arginin paradoxically relaxes pre-contracted canine intrapulmonary arteries. J Appl Physiol, 1993; 74: 549
Wang D X, ** X R, Wang W Het al. Effects of cigarette smoking on HPV and its relation to animal species and period of smoking. J Tongji Med Univ, 1992; 12: 75
Weir E K, McMurtry X, Tucker A,et al. Prostaglandin synthesis inhibitors do not decrease hypoxic pulmonary vasoconstriction. J Appl Physiol, 1976; 41: 714
Yang D S. Effects of 3, 4-Dihydroxyacetophenone on the biosynthesis of TXA and PGI in human placental villus and umbilical artery segments in vitro. Prostaglandins, 1989, 38: 497
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ullah, F., Di-xun, W. & Jie, D. Effects of 3, 4-Dihydroxyacetophenone on hypoxic vasoconstriction in isolated pulmonary and basilar arterial rings. Journal of Tongji Medical University 14, 252–256 (1994). https://doi.org/10.1007/BF02897681
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02897681