Abstract
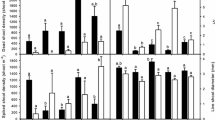
Phragmites australis (common reed), a clonal grass, has expanded from a minor component of the mid-Atlantic wetlands to a dominant species. It has been suggested that invasive populations ofPhragmites are an exotic haplotype responsible for the dramatic increase in the distribution of the species. We used field observations and measurements and a greenhouse assay to compare native (haplotype F) and exotic (haplotype M) populations, growing adjacent to one another in a brackish marsh near Odessa, Delaware. In the marsh, shoots of the exotic strain emerged from the rhizomes earlier than those of the native and by March there was an order of magnitude more new shoots of the exotic strain than the native. In August, the exotic strain was 30% taller than the native, had twice the leaf biomass, and twice the total biomass. Nine of ten morphological and biomass characteristics measured differed significantly between the native and exotic strains. A greenhouse assay was conducted by planting rhizomes collected in March in shallow trays and growing them for 70 d followed by shoot harvest (Harvest 1). Rhizomes were measured, replanted, and grown for 35 d after which time they were measured and shoots were harvested (Harvest 2). At Harvest 1, shoot height was approximately 80% greater in the exotic strain, shoot biomass was three times higher, aboveground to belowground biomass ratio was twice as high, and rhizome internode length was 50% greater in the exotic strain than the native. These traits, in addition to number of shoots, were also greater in the exotic strain at Harvest 2. The number of rhizome buds at Harvest 1 was three times greater in the native than in the exotic strain. The greater number of rhizome buds in the native would seem to be an advantage, but it did not result in more shoot production. Buds were maintained in an inactive state that does not allow this strain to compete well in a wetland environment inhabited by a more efficient spreader. The earlier emergence of new shoots from the rhizomes, the greater aboveground structure, the greater rhizome internode length, and the quick transition of rhizome buds to shoot or rhizome explain in part the exotic strain's advantage over the native and the mechanisms for its invasive nature.
Similar content being viewed by others
Literature Cited
Amsberry, L., M. A. Baker, P. J. Ewanchuk, andM. D. Bertness. 2000. Clonal integration and the expansion ofPhragmites australis.Ecological Applications 10:1110–1118.
Bertness, M. D., P. J. Ewanchuk, andB. R. Silliman. 2002. Anthropogenic modification of New England salt marsh landscapes.Proceedings of the National Academy of Sciences of the United States of America 99:1395–1398.
Chambers, R. M., L. A. Meyerson, andK. Saltonstall. 1999. Expansion ofPhragmites australis into tidal wetlands of North America.Aquatic Botany 64:261–273.
Clevering, O. A., H. Brix, andJ. Lukavska. 2001. Geographic variation in growth responses inPhragmites australis.Aquatic Botany 69:89–108.
Hansen, R. M. 1978. Shasta ground sloth food habits, Rampart Cave, Arizona.Paleobiology 4:302–319.
Hauber, D. P., D. A. White, S. P. Powers, andF. R. Defrancesch. 1991. Isozyme Variation and Correspondence with Unusual Infrared Reflectance Patterns inPhragmites australis (Poaceae).Plant Systematics and Evolution 178:1–8.
Hellings, S. E. andJ. L. Gallagher. 1992. The effects of salinity and flooding onPhragmites australis.Journal of Applied Ecology 29:41–49.
Kuhl, H., H. Koppitz, H. Rolletschek, andJ. G. Kohl. 1999. Clone specific differences in aPhragmites australis stand I. Morphology, genetics and site description.Aquatic Botany 64:235–246.
Lessmann, J. M., H. Brix, V. Bauer, O. A. Clevering, andF. A. Comin. 2001. Effect of climatic gradients on the photosynthetic responses of fourPhragmites australis populations.Aquatic Botany 69:109–126.
Lissner, J. andH. H. Schierup. 1997. Effects of salinity on the growth ofPhragmites australis.Aquatic Botany 55:247–260.
Lynch, E. A. andK. Saltonstall. 2002. Paleoecological and genetic analyses provide evidence for recent colonization of nativePhragmites australis populations in a Lake Superior wetland.Wetlands 22:637–646.
Niering, W. A., R. S. Warren, andC. G. Weymouth. 1977. Our dynamic tidal marshes: Vegetation changes as revealed by peat analysis. Connecticut, Arboretum Bulletin No. 22. The Connecticut Arboretum, New London, Connecticut.
Pauca-Comanescu, M., O. A. Clevering, J. Hanganu, andM. Gridin. 1999. Phenotypic differences among ploidy levels ofPhragmites australis growing in Romania.Aquatic Botany 64:223–234.
Rolletschek, H., A. Rolletschek, H. Kuhl, andJ. G. Kohl. 1999. Clone specific differences in aPhragmites australis stand. II. Seasonal development of morphological and physiological characteristics at the natural site and after transplantation.Aquatic Botany 64:247–260.
Roman, C. T., W. A. Niering, andR. S. Warren. 1984. Salt-marsh vegetation change in response to tidal restriction.Environmental Management 8:141–149.
Rooth, J. E., J. C. Stevenson, andJ. C. Cornwall. 2003. Increased sediment accretion rates following invasion byPhragmites australis: The role of litter.Estuaries 26:475–483.
Saltonstall, K. 2002. Cryptic invasion by a non-native genotype of the common reed,Phragmites australis, into North America.Proceedings of the National Academy of Sciences of the United States of America 99:2445–2449.
Saltonstall, K. 2003a. Genetic variation among North American populations ofPhragmites australis: Implications for management.Estuaries 26:444–451.
Saltonstall, K. 2003b. A rapid method for identifying the origin of North AmericanPhragmites populations using RFLP analysis.Wetlands 23:1043–1047.
Saltonstall, K., P. M. Peterson, andR. J. Soreng. 2004. Recognition ofPhragmites australis subsp. americanus (Poaceae: Arundinoideae) in North America: Evidence from morphological and genetic analyses.Sida Contributions to Botany 21:683–692.
Seliskar, D. M. 1998. Natural and tissue culture-generated variation in the salt marsh grassSporobolus virginicus: Potential selections for marsh creation and restoration.Hortscience 33:622–625.
Seliskar, D. M. andJ. L. Gallagher. 1992. Accelerating salt marsh functional development through plant genotype selection.In INTECOL's IV International Wetlands Conference, Columbus, Ohio. Ohio State University, Columbus, Ohio.
Seliskar, D. M. andJ. L. Gallagher. 2000. Exploiting wild population diversity and somaclonal variation in the salt marsh grassDistichlis spicata (Poaceae) for marsh creation and restoration.American Journal of Botany 87:141–146.
Seliskar, D. M., J. L. gallagher, D. M. Burdick, and L. A. Mutz. 2002. The regulation of ecosystem functions by ecotypic variation in the dominant plant: ASpartina alterniflora saltmarsh case study.Journal of Ecology 90:1–11.
Seliskar, D. M., K. E. Smart, B. T. Higashikubo, andJ. L. Gallagher. 2004. Seedling sulfide sensitivity among plant species colonizingPhragmites-infested wetlands.Wetlands 24:426–433.
Tucker, G. C. 1990. The genera of Arundinoideae (Gramineae) in the southeastern United States.Journal of the Arnold Arboretum 71:145–177.
White, D. A., D. P. Hauber, andC. S. Hood. 2004. Clonal differences inPhragmites australis from the Mississippi river delta.Southeastern Naturalist 3:531–544.
Whitham, T. G., W. P. Young, G. D. Martinsen, C. A. Gehring, J. A. Schweitzer, S. M. Shuster, G. M. Wimp, D. G. Fischer, J. K. Bailey, R. L. Lindroth, S. Woolbright, andC. R. Kuske. 2003. Community and ecosystem genetics: A consequence of the extended phenotype.Ecology 84:559–573.
Wijte, A. andJ. L. Gallagher. 1996a. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. I. Different germination strategies ofSpartina alterniflora andPhragmites australis (Poaceaei).American Journal of Botany 83:1337–1342.
Wijte, A. andJ. L. Gallagher. 1996b. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. 2. Early seedling development advantage ofSpartina alterniflora overPhragmites australis (Poaceae).American Journal of Botany 83:1343–1350.
Sources of Unpublished Materials
Meadows, R. personal communication. Delaware Department of natural Resources and Environmental Control, 2430 Old County Road, Glasgow, Delaware 19702.
Saltonstall, K. personal communication. Horn Point Laboratory, University of Maryland Center for Environmental Science, P. O. Box 775, 2020 Horns Point Road, Cambridge, Maryland 21613.
Straub, P. personal communication. Natural Sciences and Mathematics, The Richard Stockton College of New Jersey, P. O. Box 195, Pomona, New Jersey 08240.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
League, M.T., Colbert, E.P., Seliskar, D.M. et al. Rhizome growth dynamics of native and exotic haplotypes ofPhragmites australis (Common reed). Estuaries and Coasts: J ERF 29, 269–276 (2006). https://doi.org/10.1007/BF02781995
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02781995