Abstract
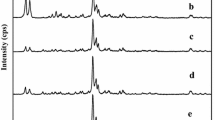
At the temperature of 90°C and under atmospheric pressure, growth kinetics of high silica ZSM-5 was investigated through a long induction, nucleation and crystal growth periods. It was found the entire crystallization mechanism of ZSM-5 seems to be the combined process of the nucleation via solid-solid transformation, intergrowth among seed crystals and the normal growth in the reaction mixture. Nuclei were initially formed on the Si-rich surface of the amorphous intermediates, indicating that the reaction of TPA with Si species was prior to that with Al species. As the reaction time proceeded, various types of intergrowth among the seed crystals were observed along with the crystals growing independently. The intergrowth seems to play a role for forming typical ZSM-5 crystal shapes. And then ZSM-5 crystals further grew in the reaction mixture, so that the bulk Si/Al2 ratio of crystals approached that of the initial reaction mixture.
Similar content being viewed by others
References
Brinker, C. J. and Scherer, G. W.,“SOL-GEL SCIENCE”, Academic Press, London, 1990.
Ciric, J.,“Kinetics of Zeolite A Crystallization”,J. Colloidal Interface Sci.,28, 315 (1968).
Cournoyer, R. A., Kranich, K. L. and Sand, L. B.,“Zeolite Crystallization Kinetics Related to Dissolution Rates of Quartz Reaetant”.J. Phys. Chem.,79, 1578 (1975).
Derouane, E. G., Detremmerie, S., Gabelica, Z. and Blom, N.,“Synthesis and Characterization of ZSM-5 Type Zeolites I. Physico-Chemical Properties of Precursors and Intermediates”,App, Cat.,1, 201(1981).
Kerr, G. T.,“Chemistry of Crystalline Aluminosilicates. I. Factors Affecting The Fomation of Zeolite A”,J. Phys. Chem.,70, 1047 (1966).
Kingery, W. D., Bowen, H. K. and Uhlmann, D. R.,“Introduction to Ceramics”, John Wiley & Sons, New York, 1976.
Konatowski, J.,“Growth of Large Crystals of ZSM-5 Zeolite”,Zeolites,8, 77(1988).
Jacobs, D. A., Derouane, E. G. and Weitkamp, J.,“Evidence for X-Ray-amorphous Zeolites”,J. Chem. Sci., Chem. Commun., 591 (1981).
Lee, K. H.,“A Study on the Synthesis Mechanism and Catalytic Performance of Pentasil Type Zeolite”, Ph. D. Dissertation, Korea Advanced Institute of Science and Technology, Taejon, Korea, 1992.
Lermar, H., Draeger, M., Steffen, J. and Unger, K. K.,“Synthesis and Structure Finement of ZSM-5 Single Crystals”,Zeolites,5, 131(1985).
Lowe, B. M.,“An Equilibrium Model for The Crystallization of High Silica Zeolites”,Zeolites,3, 300(1983).
McNicol, R. D., Pott, G. T. and Loos, K. R.,“Spectroscopic Studies of Zeolite Synthesis”,J. Phys., Chem.,70, 1047 (1966).
Minotova, S., Veltchev, V. and Kanev, I.,“A Correlation between the Fundamental Properties of Templates and the Kinetics of ZSM-5 Crystallization”,Zeolites,13, 102(1993).
Narita, E., Sato, K., Yatabe, N. and Okabe, T.,“Synthesis and Crystal Growth of Zeolite ZSM-5 from Sodium Aluminosilicate Systems Free of Organic Templates”,Ind. Eng. Prod. Res. Dev.,24, 507 (1985).
Padovan, M., Leofanti, G., Solari, M. and Moretti, E.,“Studies on the ZSM-5 Zeolite Formation”,Zeolites,4, 295(1984).
Suzuki, K., Kiyozumi, Y., Matsuzaki, K. and Shin, S.,“Influence of the Synthesis Conditions of H-ZSM-5 on Its Physical Properties and Catalytic Activity in Methanol Conversion: the Water Content of the Reaction Mixture”,App. Cat.,35, 401(1987).
Suzuki, K., Kiyozumi, Y., Matsuzaki, K. and Shin, S.,“Effect of Crystallization Time on the Physicochemical and Catalytic Properties of a ZSM-5 Type Zeolite”,App. Cat.,42, 35 (1988).
Szostak, R.,“Molecular Sieves-Principles of Synthesis and Identification”, Van Nostrand Reinhold Co., New York, NY, 1989.
Ueda, S., Murata, H. and Koizumi, M.,“Crystallization of Mordenite from Aqueous Solutions”.Amer. Miner.,65, 1012(1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kiyozumi, Y., Shin, S., Shul, Y.G. et al. Crystal growth of high silica ZSM-5 at low temperature synthesis conditions. Korean J. Chem. Eng. 13, 144–149 (1996). https://doi.org/10.1007/BF02705901
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705901