Summary
The influence of isoproterenol on myocardial performance and energetics was investigated in normal guinea pig myocardium and in patients with normal left ventricular function.
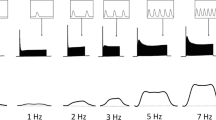
The in vitro experiments were performed by simultaneous isometric force and heat measurements using sensitive antimony-bismuth thermopiles. Following the application of isoproterenol (10−8 M) isometric peak twitch tension and tension-time integral increased significantly by 185% and 142%, respectively. Tension-independent heat which reflects high energy phosphate hydrolysis of excitation-contraction coupling increased by 183%. Tension-dependent heat reflecting the high energy phosphate hydrolysis of the crossbridges increased by 417%. The ratio of tension-dependent heat to tension-time integral increased by 131%. The recovery/initial heat ratio, reflecting the efficiency of the recovery metabolism, and the resting metabolism did not significantly change.
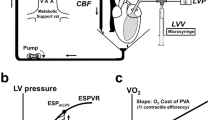
In the patients the effect of isoproterenol on myocardial energetics was evaluated in terms of myocardial oxygen consumption per left ventricular systolic stress-time integral and external myocardial efficiency. Following isoproterenol administration, left ventricular systolic stress-time integral decreased by 49% due to reductions in end-diastolic pressure, end-diastolic volume and duration of systole. Pressure-volume work remained unchanged. Myocardial oxygen consumption per minute increased in proportion to heart rate. The ratio of myocardial oxygen consumption per beat to left ventricular systolic stress-time integral increased significantly by 95%. External myocardial efficiency was unaltered.
Thus, isoproterenol increases the energy turnover of excitation-contraction coupling and increases the energy consumption of the crossbridges disproportionately to developed tension-time integral in the guinea pig heart. Likewise, in the working human heart, the increase in oxygen consumption per left ventricular systolic stress-time integral is considered to represent the isoproterenol induced changes in excitation contraction coupling and crossbridge energetics.
Similar content being viewed by others
References
Allen DG, Kurihara S (1980) Calcium transients in mammalian ventricular muscle. Eur Heart J 1:5–15
Alpert NR, Mulieri LA (1982) Increased myothermal economy of isometric force generation in compensated cardiac hypertrophy induced by pulmonary artery constriction in the rabbit. Circ Res 50:491–500.
Alpert NR, Mulieri LA (1984) Hypertrophic adaptation of the heart to stress: A myothermal analysis. In: Zak R (ed) Growth of the heart in health and disease. Raven Press, New York, pp 363–379
Alpert NR, Blanchard EM, Mulieri LA (1989) Tension-independent heat in rabbit papillary muscle. J Physiol (in press)
England PJ (1986) The phosphorylation of cardiac contractile proteins. In: Rupp H (ed) The regulation of heart function. Basic concepts and clinical applications. Thieme, Stuttgart New York, pp 223–233
Gibbs CL, Gibson WR (1972) Isoprenaline, propranolol, and the energy output of rabbit cardiac muscle. Cardiovasc Res 6:508–515
Gibbs CL (1978) Cardiac energetics. Physiol Rev 58:174–254
Hasenfuss G, Holubarsch Ch, Just H, Blanchard E, Mulieri LA, Alpert NR (1987) Energetic aspects of inotropic interventions in rat myocardium. Basic Res Cardiol 82 [Suppl. 2]:252–259
Hasenfuss G, Holubarsch Ch, Blanchard EM, Mulieri LA, Alpert NR (1989) Influence of inotropic interventions on crossbridge economy during isometric force development in guinea pig papillary muscles. Biophys J 55:44310
Hasenfuss G, Holubarsch Ch, Heiss HW, Meinertz Th, Bonzel T, Wais U, Lehmann M, Just H (1989) Myocardial energetics in patients with dilative cardiomyopathy. Influence of nitroprusside and enoximone. Circulation (in press)
Holubarsch Ch, Hasenfuss G, Heiss HW, Bonzel T (1987) The relation between myocardial oxygen consumption and systolic stress-development in patients with normal hearts and with idiopathic dilative cardiomyopathy. Circulation 76 [Suppl IV]:161
Hoh JFY, Rossmanith GH, Kwan LJ, Hamilton AM (1988) Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated pertubation analysis. Circ Res 62:452–461
Katz A (1983) Cyclic adenosine monophosphate effects on the myocardium: a man who blows hot and cold with one breath. J Am Col Cardiol 2:143–149
Klocke FJ, Kaiser GA, Ross JR, Braunwald E (1965) Mechanism of increase of myocardial oxygen uptake produced by catecholamines. Am J Physiol 209:913–918
Krasnow N, Rolet EL, Yurchak PM, Hood WB, Gorlin R (1964) Isoprotereol and cardiovascular performance. Am J Med 37:514–525
Laskey WK, Reichek N, Sutton MS, Untereker WJ, Hirshfeld JW (1983) Myocardial oxygen consumption in left ventricular hypertrophy and its relation to left ventricular mechanics. Am J Cardiol 52:852–858
Lekven J, Brunsting LA, Jessen ME, Abd-Elfatth AS, Doherty N, Wechsler AS (1988) Myocardial oxygen use during epinephrine administration to ischemically injured canine hearts. Circulation 78 [Suppl III]:125–136
Mirsky I (1979) Elastic properties of the myocardium: a quantitative approach with physiological and clinical applications. In: Berne RM (ed) Handbook of physiology. The cardiovascular system. American Physiological Society, Washington DC, pp 497–531
Mulieri LA, Luhr G, Trefry J, Alpert NR (1977) Metal-film thermopiles for use with rabbit right ventricular papillary muscles. Am J Physiol 2:C146–156
Rackley CE, Dodge HT, Coble YD, Haya RE (1964) A method for determining left ventricular mass in man. Circulation 29:666–671
Rau G (1969) Messung der Koronardurchblutung mit der Argon-Fremdgasmethode. Arch Kreislauf-Forsch 58:322–398
Reuter H, Scholz H (1977) The regulation of calcium conductance of cardiac muscle by adrenaline. J Physiol 264:49–62
Rooke GA, Feigl EO (1982) Work as a correlate of canine left ventricular oxygen consumption, and the problem of catecholamine oxygen wasting. Circ Res 50:273–286
Rüegg JC (1986) The vertebrate heart: Modulation of calcium control. In Ruegg JC (ed) Calcium in muscle activation. Springer, Berlin Heidelberg New York London Paris Tokyo, pp 165–201
Sandler H, Dodge HT (1968) The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am Heart J 75:325–334
Sonnenblick EH, Ross J, Covell JW, Kaiser GH, Braunwald E (1965) Velocity of contraction as a determinant of myocardial oxygen consumption. Am J Physiol 209:919–927
Suga H, Hisano R, Goto Y, Yamada O, Igarashi Y (1983) Effect of positive inotropic agents on the relation between oxygen consumption and systolic pressure volume area in canine left ventricle. Circ Res 53:306–318
Weber KT, Janicki (1977) Myocardial oxygen consumption: The role of wall force and shortening. Am J Physiol 233:421–430
Winegrad S (1984) Regulation of cardiac contractile proteins. Correlation between physiology and biochemistry. Circ Res:565–514
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasenfuss, G., Holubarsch, C., Blanchard, E.M. et al. Influence of isoproterenol on myocardial energetics. Experimental and clinical investigations. Basic Res Cardiol 84, 147–155 (1989). https://doi.org/10.1007/BF02650354
Issue Date:
DOI: https://doi.org/10.1007/BF02650354