Abstract
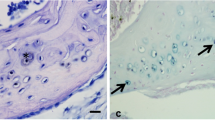
Correlated studies were performed with light and electron microscopy, and backscattered electron image in conjunction with X-ray microanalysis, of lanthanum-incubated epiphyseal cartilage of the young rat. The hallmark of this procedure is the appearance of LaP electrondense deposits (not present in control sections) in precise sites of the hypertrophic zone. The ultrastructural study revealed a dual nature of these sites: “dense matrix vesicles” and “focal filament aggregates.” The dense matrix vesicles are a specific type of matrix vesicle with the intrinsic capacity of precipitating LaP mineral, as soon as they originate from the hypertrophic chondrocytes. Furthermore, the matrix vesicles were found to be heterogeneous because lanthanum-devoid, “light matrix vesicles” were also present. The focal filament aggregates, which were not recognized in unstained sections and in controls, are apparently focal concentrations of proteoglycans with high lanthanum binding capacity, although the presence in them of other components (e.g., type X collagen, C-propeptide of type II collagen) cannot be excluded. They were in close connection with the light matrix vesicles in the upper hypertrophic zone, and were loaded with a variable quantity of LaP irregular electron-dense deposits in the lower hypertrophic zone. These irregular deposits are similar to, but distinct from, calcification nodules. The lanthanum incubation method indirectly detects the matrix Ca-binding components (which bind La ions), and the calcification initiation sites (which precipitate a LaP-mineral phase). A sequence is proposed of successive steps of LaP nucleation within the focal filament aggregates, which possibly mimics calcium phosphate deposition. Such a sequence seems to require the participation not only of dense matrix vesicles, but also of the filamentous components of the focal aggregates, possibly together with the activity of alkaline phosphatase.
Similar content being viewed by others
References
Morris DC, Appleton J (1984) The effects of lanthanum on the ultrastructure of hypertrophic chondrocytes and the localization of lanthanum precipitates in condylar cartilages of rats fed on normal and rachitogenic diets. J Histochem Cytochem 32: 239–247
Bonucci E, Reurink J (1978) The fine structure of decalcified cartilage and bone: a comparison between decalcification procedures performed before and after embedding. Calcif Tissue Res 25:179–190
Gomez S, Boyde A (1994) Correlated alkaline phosphatase histochemistry and quantitative backscattered electron imaging in the study of rat incisor ameloblasts and enamel mineralization. Microsc Res Technique 29:29–36
Arsenault AL, Hunziker EB (1988) Electron microscopic analysis of mineral deposits in the calcifying epiphyseal growth plate. Calcif Tissue Int 42:119–126
Landis WJ, Glimcher MJ (1982) Electron optical and analytical observations of rat growth plate cartilage prepared by ultracyomicrotomy: the failure to detect a mineral phase in matrix vesicles and the identification of heterodispersed particles as the initial solid phase of calcium phosphate deposited in the extracellular matrix. J Ultrastruct Res 78:227–268
Wu LNY, Yoshimori T, Genge BR, Sauer GR, Kirsch T, Ishikawa Y, Wuthier RE (1993) Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem 268: 25084–25094
Kirsch T, Ishikawa Y, Mwale F, Wuthier RE (1994) Roles of the nucleational core complex and collagens (types II and X) in calcification of growth plate cartilage matrix vesicles. J Biol Chem 269:20103–20109
Sela J, Schwartz Z, Swain LD, Boyan BD (1992) The role of matrix vesicles in calcification. In: Bonucci E (ed) Calcification in biological systems. CRC Press, Boca Raton, pp. 73–105
Wuthier RE (1977) Electrolytes of isolated epiphyseal chondrocytes, matrix vesicles, and extracellular fluid. Calcif Tissue Res 23:125–133
Pollesello P, de Bernard B, Grandolfo M, Paoletti S, Vittur F, Kvam BJ (1991) Energy state of chondrocytes assessed by31P-NMR studies of preosseous cartilage. Biochem Biophys Res Comm 180:216–222
Wuthier RE (1992) Matrix vesicles: formation and function. Mechanisms in membrane/matrix-mediated mineralization. In: Slavkin H, Price P (eds) Chemistry and biology of mineralized tissues. Excerpta Medica, Amsterdam, pp 143–152
Bonucci E, Silvestrini G, Bianco P (1992) Extracellular alkaline phosphatase activity in mineralizing matrices of cartilage and bone: ultrastructural localization using a cerium-based method. Histochemistry 97:323–327
Lewinson D, Toister Z, Silbermann M (1982) Quantitative and distributional changes in the activity of alkaline phosphatase during the maturation of cartilage. J Histochem Cytochem 32:261–269
Warner GP, Hubbard HL, Lloyd GC, Wuthier RE (1983)32Pi- and45Ca-metabolism by matrix vesicle-enriched microsomes prepared from chicken epiphyseal cartilage by isosmotic Percoll density-gradient fractionation. Calcif Tissue Int 35: 327–338
Shepard N (1992) Role of proteoglycans in calcification. In: Bonucci E (ed) Calcification in biological systems. CRC Press, Boca Raton, pp 41–58
Takagi M (1990) Ultrastructural cytochemistry of cartilage proteoglycans and their relation to the calcification process. In: Bonucci E, Motta PM (eds) Ultrastructure of skeletal tissues. Kluwer Academic Publishers, Boston, pp 111–127
Ehrlich MG, Armstrong AL, Neuman RG, Davis MW, Mankin HJ (1982) Patterns of proteoglycan degradation by a neutral protease from human growth-plate epiphyseal cartilage. J Bone Joint Surg Am 64:1350–1354
Dean DD, Schwartz Z, Muniz OE, Gomez R, Swain LD, Howell DS, Boyan BD (1992) Matrix vesicles are enriched in metalloproteinases that degrade proteoglycans. Calcif Tissue Int 50:342–349
Poole R, Matsui Y, Hinek A, Lee ER (1989) Cartilage macromolecules and the calcification of cartilage and bone. Anat Rec 224:167–179
Schmid TM, Linsenmayer TF (1990) Immunoelectron microscopy of type X collagen: supramolecular forms within embryonic chick cartilage. Dev Biol 138:53–62
Kwan APL, Cummings CE, Chapman JA, Grant ME (1991) Macromolecular organization of chicken type V collagen in vitro. J Cell Biol 114:597–604
Kirsch T, von der Mark K (1991) Ca2+ binding properties of type X collagen. FEBS Lett 294:149–152
Wu LNY, Genge BR, Lloyd GC, Wuthier RE (1991) Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. J Biol Chem 266:1195–1203
Kirsch T, Wuthier RE (1994) Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J Biol Chem 269:11462–11469
Wu LNY, Genge BR, Wuthier RE (1991) Association between proteoglycans and matrix vesicles in the extracellular matrix of growth plate cartilage. J Biol Chem 266:1187–1194
Poole AR (1991) The growth plate: cellular physiology, cartilage assembly and mineralization. In: Hall B, Newman S (eds) Cartilage: molecular aspects. CRC Press, Boca Raton, pp 179–211
Bonucci E (1967) Fine structure of early cartilage calcification. J Ultrastruc Res 20:33–50
Bonucci E, Silvestrini G (1992) Immunohistochemical investigation on the presence of chondroitin sulfate in calcification nodules of epiphyseal cartilage. Eur J Histochem 36:407–422
Bonucci E, Silvestrini G, Di Grezia R (1988) The ultrastructure of the organic phase associated with the inorganic substance in calcified tissues. Clin Orthop Rel Res 233:243–261
Bonucci E, Silvestrini G, Di Grezia R (1988) Histochemical properties of the “crystal ghosts” of calcifying epiphyseal cartilage. Connect Tissue Res 22:43–50
Althoff J, Quint P, Krefting E-R, Höhling HJ (1982) Morphological studies on the epiphyseal growth plate combined with biochemical and X-ray microprobe analyses. Histochemistry 74:541–552
de Bernard B, Bianco P, Bonucci E, Costantini M, Lunazzi GC, Martinuzzi P, Modricky C, Moro L, Panfili E, Pollesello P, Stagni N, Vittur F (1986) Biochemical and immunohistochemical evidence that in cartilage an alkaline phosphatase is a Ca2+-binding glycoprotein. J Cell Biol 103:1615–1623
Vittur F, Stagni N, Moro L, de Bernard B (1984) Alkaline phosphatase binds to collagen: a hypothesis on the mechanism of extravesicular mineralization in epiphyseal cartilage Experientia 40:836–837
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gomez, S., Lopez-Cepero, J.M., Silvestrini, G. et al. Matrix vesicles and focal proteoglycan aggregates are the nucleation sites revealed by the lanthanum incubation method: A correlated study on the hypertrophic zone of the rat epiphyseal cartilage. Calcif Tissue Int 58, 273–282 (1996). https://doi.org/10.1007/BF02508648
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02508648