Abstract
l-Tryptophan (Trp) was widely used as a natural tool for the support of serotonin-mediated brain functions and as a challenge probe for the assessment of serotonin-mediated neuroendocrine responses. The metabolic fate of the administered Trp and the kinetics of the accumulation of Trp metabolites in the circulation, however, have never thoroughly been investigated.
This study describes the time- and dose-dependent alterations in the plasma levels of various Trp metabolites and large neutral amino acids after the infusion of Trp to healthy young men (1, 3 and 5 g; placebo-controlled, double-blind, cross-over study during day- and night-time).
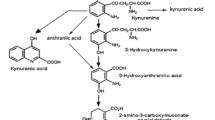
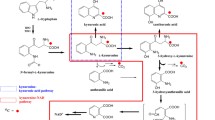
The major Trp metabolites (kynurenine, indole acetic acid and indole lactic acid) in plasma increased dose-dependently but rather slowly after Trp administration to reach their maximal plasma levels (up to 10-fold after the 5 g dose) at about 3 h p.i., and remained at an elevated level (about 5-fold) for up to 8 h. N-acetyl-Trp and 5-hydroxy-Trp rose rapidly and massively after Trp infusions, at the 5 g dose more than 200- and 20-fold, respectively, and declined rapidly to about 5-fold baseline levels within 2 h. Whole blood serotonin levels were almost unaffected by the Trp infusions. A rather slow increase of 5-hydroxyindole acetic acid was seen, reaching maximum values (3-fold at the 5 g dose) at about 2 h after the infusion of Trp. Additionally, a dose-dependent rise of circulating melatonin was observed afterl-Trp infusions. The administration ofl-Trp caused a depletion of the concentrations of the other large neutral amino acids and a dose dependent decrease of the ratio between plasma tyrosine and the sum of the plasma concentrations of the other large neutral amino acids. Apparently, none of the existing pathways of peripheral Trp metabolism is saturated by its substrate, Trp in men. At least some of the central effects reported afterl-Trp administration may be mediated by the Trp-stimulated formation of neuroactive metabolites or by the decreased availability of tyrosine for catecholamine synthesis.
Similar content being viewed by others
References
Aizenstein ML, Scavone C (1984) The urinary excretion of 5-hydroxyindoleacetic acid does not reflect brain levels of this metabolite of 5-hydroxytryptamine. Braz J Med Biol Res 17:323–327
Ashcroft GW, Eccleston D, Crawford TBB (1965) 5-Hydroxyindole metabolism in rat brain. A study of intermediate metabolism using the technique of tryptophan loading. J Neurochem 12:483–492
Aviram M, Cogan U, Modady S (1991) Excessive dietary tryptophan enhances plasma-lipid peroxidation in rats. Atherosclerosis 88:29–34
Belongia EA, Hedberg CW, Gleich GJ (1990) An investigation of the cause of the eosinophilia-myalgia syndrome associated with tryptophan use. N Engl J Med 323:357–365
Brainard GC, Lewy AJ, Menaker M, Fredrikson RH, Miller LS, Wellber RG, Caryone V, Hudson D (1988) Dose-response relationship between light irridance and the suppression of plasma melatonin in human volunteers. Brain Res 454:212–218
Brown RR, Lee CM, Kohler PC, Hank JA, Storer BE, Sondel PM (1989) Altered tryptophan and neopterin metabolism in cancerpatients treated with recombinant interleukin-2. Cancer Res 49:4941–4944
Byrne GJ, Lehmann LK, Kirschbaum JG, Borden EC, Lee CM, Brown RR (1986) Induction of tryptophan-degradation in vitro and in vivo: a γ-interferon-stimulated activity. J Interferon Res 6:389–396
Carboni E, Cadoni C, Tanda GL, Dichiara G (1989) Calcium-dependent, tetrodotoxin-sensitive stimulation of cortical serotonin release after a tryptophan load. J Neurochem 53:976–978
Charlton KG, Johnson TD, Mamed AT, Clarke DE (1983) Cardiovascular actions of kynuramine and 5-hydroxykynuramine in pithed rats. J Neural Transm 57:199–211
Christen S, Peterhans E, Stocker R (1990) Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci USA 87:2506–2510
Cowen PJ (1988) Neuroendocrine responses to tryptophan as an index of brain serotonin function. In: Huether G (ed) Amino acid availability and brain function in health and disease. NATO ASI Series, vol H20, Springer, Berlin Heidelberg, New York, pp 285–290
Fernstrom JD, Wurtman RJ (1971) Brain serotonin content: physiological dependence on plasma tryptophan levels. Science 173:149–151
Gal EM, Young RB, Sherman AD (1978) Tryptophan loading: consequent effects on the synthesis of kynurenine and 5-hydroxy-indoles in rat brain. J Neurochem 31:237–244
Gearney DP, Soper N, Shepstone BJ, Goodwin GM, Cowen PJ (1991) Intravenousl-tryptophan and regional cerebral blood-flow. Biol Psychiatry 29:508–509
George CFB, Millar TW, Hanly PJ, Kryger MH (1989) The effect ofl-trytophan on daytime sleep latency in normals—correlation with blood-levels. Sleep 12:345–353
Gessa GL, Biggio G, Fadda F, Corsini GU, Tagliamonte A (1974) Effect of the oral administration of Trp-free amino acid mixtures on serum tryptophan, brain tryptophan and serotonin metabolism. J Neurochem 22:869–870
Goodwin GM, Shapiro CM, Bennie J, Dick H, Carroll S, Fin KG (1989) The neuroendocrine responses and psychological effects of infusion ofl-tryptophan in anorexia nervosa. Psychol Med 19:857–864
Hajak G, Huether G, Blanke J, Bloemer M, Freyer C, Poeggeler B, Reimer A, Rodenbeck A, Schulz-Varszegi M, Rüther E (1991) The influence of intravenousl-tryptophan on plasma melatonin and sleep in men. Pharmacopsychiatry 24:17–20
Hajak G, Huether G, Rodenbeck A, Rüther E (1992) Endocrine and sleep-inducing properties ofl-tryptophan in men In: Lehnert H et al (eds) Endocrine and nutritional control of basic biological functions. Hogrefe and Huber, Bern, Stuttgart, Toronto (in press)
Hartmann EL (1986) Effect ofl-tryptophan and other amino-acids on sleep. Nutr Rev 44:70–73
Heyes MP, Markey SP (1988) Quantification of quinolinic acid in rat brain, whole blood and plasma by GCMS: effects of systemicl-Trp administration on brain and blood quinolinic acid concentrations. Anal Biochem 174:349–359
Huether G, Lajtha A (1991) Changes in free amino acid concentrations in serum, brain, and CSF throughout embryogenesis. Neurochem Res 16:145–150
Huether G, Poeggeler B, Reimer A, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: Evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci 51:945–953
Hussain MN, Sirek A, Cukerman E, Sirek OV (1985) The effect of tryptophan on biogenic amines in the hepatic portal circulation of the dog. Can J Physiol 63:863–866
Idzikowski C, Mills FJ, Glennard R (1986) 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res 378 164–168
Jones ML, Kimbrough TD, Weekley LB (1985) Disturbances of tryptophan metabolism in mice acutely deprived of tryptophan. Ann Nutr Metab 29:209–215
Juorio AV, Paterson IA (1990) Tryptamine may couple dopaminergic and serotonergic transmission in the brain. Gen Pharmacol 21:613–616
Körner E, Bertha G, Flooh E, Reinhart B, Wolf R, Lechner H (1986) Sleep-inducing effect ofl-tryptophan. Eur Neurol 25:75–81
Lapin JP (1988) Behavioral and convulsant effects of kynurenines. In: Stone TW (ed) Quinolinic acid and the kynurenines. CRC Press, Boca Raton, Florida, pp 193–211
Lee MA, Nash JF, Barnes M, Meltzer HY (1991) Inhibitory effect of ritanserin on the 5-hydroxytryptophan-mediated cortisol, ACTH and prolactin secretion in humans. Psychopharmacology 103:258–264
Liebermann HR (1986) Behavior, sleep and melatonin. J Neural Transm (Suppl) 21:233–241
Maestroni GJ, Conti MA, Pierpalle W (1987) The pineal gland and the circadian opiatergic, immunoregulatory role of melatonin. Ann NY Acad Sci 496:67–77
Marsden CA, Curzon G (1978) The contribution of tryptamine to the behavioural effects ofl-Trp in tranylcypramine-treated rats. Psychopharmacology 57:71–76
Marsden CA, Curzon G (1979) The role of tryptamine in behavioural effects of tranylcypramine plusl-tryptophan. Neuropharmacology 18:159–164
Masiello P, Balestrere E, Bacciola D, Bergamini E (1987) Influence of experimental diabetes on brain levels of monoamine neurotransmitters and their precursor amino acids during tryptophan loading. Acta Diabetol Lat 24:43–50
Mason R, Brooks A (1988) The electrophysiological effects of melatonin and a putative melatonin antagonist (N-acetyltryptamine) on rat suprachiasmatic neurones in vitro. Neurosci Lett 95:296–301
Mirmian M, Pevet P (1986) Effect of melatonin and 5-methoxytryptamine on sleep-wake-patterns in the male rat. J Pineal Res 3:135–141
Moir ATB, Eccleston D (1968) The effects of precursor loading in the cerebral metabolism of 5-hydroxyindoles. J Neurochem 15:1093–1108
Moja EA, Restani P, Corsii E, Stacchezzini MC, Assereto R, Galli CL (1991) Cycloheximide blocks the fall of plasma and tissue tryptophan levels after tryptophan-free amino-acid mixtures. Life Sci 49:1121–1128
Morand C, Young SN, Ervin FR (1983) Clinical response of aggressive schizophrenics to oral tryptophan. Biol Psychiatry 18:575–578
Poeggeler B, Huether G (1991) A versatile homogenous one-tube scintillation proximity radioimmunoassay for melatonin. Clin Chem 38:314–315
Poeggeler B, Reimer A, Huether G (1992) The origin of elevated plasma melatonin after administration ofl-tryptophan. In: Lehnert H et al (eds) Endocrine and nutritional control of basic biological functions. Hogrefe and Huber, Bern, Stuttgart, Toronto (in press)
Pomfret DW, Schenck KW, Fudzinski P, Cohen ML (1987) Interaction of 5-OH-kynurenamine,l-kynurenine, and kynuramine with multiple serotonin receptors in smooth muscle. J Pharmacol Exp Ther 241:465–471
Price LH, Charney DS, Delgado PL, Heninger GR (1991) Serotonin function and depression—neuroendocrine and mood responses to intravenousl-tryptophan in depressed patients and healthy comparison subjects. Am J Psychiatry 148:1518–1525
Renson J, Weissbach H, Udenfriend S (1962) Hydroxylation of tryptophan by phenylalanine hydroxylase. J Biol Chem 237:2261–2264
Rizza V, Bousquet E, Guerrera F, De Regis M (1983) Regulation of cerebral kynurenine and 5-hydroxyindole pathways during tryptophan loading. Cephalalgia 3:139–142
Schaechter JD, Wurtman RJ (1990) Serotonin release varies with brain tryptophan levels. Brain Res 532:203–210
Schneider-Helmert D, Spinweber CL (1986) Evaluation ofl-tryptophan for treatment of insomnia: a review. Psychopharmacology 89:1–7
Schwarcz T, Whetsell WO, Mangano RM (1983) Quinolonic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science 219:316–318
Schwartz DH, Hernandez L, Hoebel BG (1990) Tryptophan increases extracellular serotonin in the lateral hypothalamus of food-deprived rats. Brain Res Bull 25:803–807
Sidransky H, Sarma DSR, Bongiorno M, Berney E (1968) Effect of dietary tryptophan on hepatic ribosomes and protein synthesis in fasted mice. J Biol Chem 243:1123–1132
Sleight AJ, Marsden CA, Martin KF, Palfreyman MG (1988) Relationship between extracellular 5-hydroxytryptamine and behavior following monoamine-oxidase inhibition andl-tryptophan. Br J Pharmacol 93:303–310
Slutsker L, Hoesley FC, Miller L (1990) Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer JAMA 264:213–217
Stone TW, Connick JH (1985) Quinolinic acid and other kynurenines in the central nervous system. Neuroscience 15:597–617
Sved AF, Van Itallie DM, Fernstrom JD (1982) Studies on the antihypertensive action ofl-tryptophan. J Pharmacol Exp Ther 221:329–333
Teff KL, Young SN (1988) Effects of carbohydrate and protein administration on rat tryptophan and 5-hydroxytryptamine—differential effects on the brain, intestine, pineal, and pancreas. Can J Physiol Pharmacol 66:683–688
Thomson E, Rankin H, Ashcroft GW, Yates CM, McQueen JK, Cummings SW (1982) The treatment of depression in general practice: a comparison ofl-tryptophan, amitryptiline, and a combination ofl-tryptophan and amitryptiline with placebo. Psychol Med 12:741–751
Träskman-Bendz L, Haskett RF, Zis AP (1986) Neuroendocrine effects ofl-tryptophan and dexamethasone. Psychopharmacology 89:85–88
Van Praag HM, Lemus C, Kahn R (1987) Hormonal probes of central serotonergic activity: do they really exist. Biol Psychiatry 22:86–98
Waldhauser F, Saletu B, Trinchard-Lugan I (1990) Sleep laboratory investigations of hypnotic properties of melatonin. Psychopharmacology 100:222–226
Weissbach H, King W, Sjoersdsma A, Udenfriend S (1959) Formation of indole-3-acetic acid and tryptamine in animals. J Biol Chem 234:81–86
Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H (1989) Characteristics of interferon induced tryptophan-metabolism in human-cells in vitro. Biochim Biophys Acta 1012:140–147
Winokur A, Lindberg ND, Lucki I, Phillips J, Amsterdam JD (1986) Hormonal and behavioral effects associated with intravenousl-tryptophan administration. Psychopharmacology 88:213–219
Wurtman RJ, Fernstrom JD (1976) Control of brain neurotransmitter synthesis by precursor availability and nutritional state. Biochem Pharmacol 25:1691–1696
Woolf PD, Lee L (1976) Effect of the serotonin precursor, tryptophan, on pituitary hormone secretion. J Clin Endocrinol Metab 45:123–133
Yasui H, Takai K, Yoshida R, Hayaishi OP, Nas US (1986) Interferon enhances tryptophan-metabolism by inducing pulmonary indoleamine 2,3-dioxygenase—its possible occurrence in cancer-patients. Proc Natl Acad Sci USA 83:6622–6626
Yoshida R, Urade Y, Tokuda M, Hayashi O (1979) Induction of indoleamine 2, 3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci USA 76:4084–4086
Young SN (1986) The clinical psychopharmacology of tryptophan. In: Wurtman RJ, Wurtman JJ (eds) Nutrition and the brain, vol 7 Raven Press, New York, pp 49–88
Young SN, Gauthier S (1981) Tryptophan availability and the control of 5-hydroxytryptamine and tryptamine synthesis in human CSF. Adv Exp Med Biol 133:221–230
Young SN, St-Arnaud-McKenzie D, Sourkes TL (1978) Importance of tryptophan pyrrolase and aromatic amino acid decarboxylase in the catabolism of tryptophan. Biochem Pharmacol 27:763–767
Yuwiler A, Brammer GL, Morley JE et al. (1981) Short-term and repetitive administration of oral tryptophan in normal men. Arch Gen Psychiatry 38:619–626
Author information
Authors and Affiliations
Additional information
Part of this work was presented at the International Symposium on Future Prospects ofl-Tryptophan in Medicine, Heidelberg, Germany, November 22–23, 1991
Rights and permissions
About this article
Cite this article
Huether, G., Hajak, G., Reimer, A. et al. The metabolic fate of infusedl-tryptophan in men: possible clinical implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psychopharmacology 109, 422–432 (1992). https://doi.org/10.1007/BF02247718
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02247718