Summary
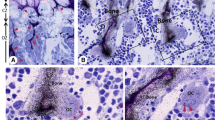
The cyclic AMP and cyclic GMP concentrations of alveolar bone of control and PTE-treated cats were measured by chemical and immunohistochemical methods. In the PTE-treated animals, alveolar bone osteoblasts stained intensely for cAMP, but very weakly for cGMP; the periodontal ligament (PDL) cells stained for cAMP similarly to the controls, but some PDL cells stained more intensely for cGMP than their controls; osteocytes stained for cAMP with greater intensity than in the controls; osteoclasts stained intensely for both cyclic nucleotides. We found that bone samples taken from animals 20 and 60 min after administration of PTE contained twice the amount of cAMP, and almost three times the amount of cGMP observed in the controls. These results indicate that the cellular source of bone cyclic nucleotides in PTE-treated animals varies as to cell type, and therefore in bone and PDL the functions mediated by cAMP are not necessarily antagonistic to those mediated by cGMP.
Similar content being viewed by others
References
Brooker, T.L., Jr., Appleman, M.M.: The assay of adenosine 3′,5′-cyclic monophosphate and guanosine 3′,5′-cyclic monophosphate in biological materials by enzymatic radio-isotopic displacement. Biochemistry7, 4177–4181 (1968)
Chase, L.R., Aurbach, G.D.: The effect of parathyroid hormone on the concentration of adenosine 3′,5′-monophosphate in skeletal tissue in vitro. J. Biol. Chem.245, 1520–1526 (1970)
Davidovitch, Z., Montgomery, P.C., Eckerdal, O., Gustafson, G.T.: Demonstration of cyclic GMP in bone cells by immunohistochemical methods. Calcif. Tiss. Res.19, 305–315 (1976)
Davidovitch, Z., Montgomery, P.C., Eckerdal, O., Gustafson, G.T.: Cellular localization of cyclic AMP in periodontal tissues during experimental tooth movement in cats. Calcif. Tiss. Res.19, 316–329 (1976)
Davidovitch, Z., Montgomery, P.C., Shanfeld, J.L.: Guanosine 3′,5′-monophosphate in bone: Microscopic visualization by an immunohistochemical technique. Calcif. Tiss. Res.24, 73–79 (1977).
DeLange, R.J., Kemp, R.G., Riley, W.D., Cooper, R.A., Krebs, E.G.: Activation of skeletal muscle phosphorylase kinase by adenosine triphosphate and adenosine 3′,5′-monophosphate. J. Biol. Chem.243, 2200–2208 (1968)
Diamantstein, T., Ulmer, A.: Effect of cyclic nucleotides on DNA synthesis in mouse lymphoid cells. Immun. Commun.4, 51–62 (1975)
Diamantstein, T., Ulmer, A.: Regulation of DNA synthesis by guanosine-5′-diphosphate, cyclic guanosine-3′,5′-monophosphate, and cyclic adenosine-3′,5′-monophosphate in mouse lymphoid cells. Exp. cell res.93, 309–314 (1974)
Dietze, G., Hepp, K.D.: Effect of 3′,5′-AMP on calcium-activated ATPase in rat heart sarcolemma. Biochem. Biophys. Res. Commun.46, 269–278 (1972)
Eckerdal, O.: Tomography of the temporomandibular joint. Acta Radiologica, supplement329, 1–107 (1973)
Entmann, M.L., Levey, G.S., Epstein, S.E.: Mechanism of action of epinephrine and glucagon on the canine heart: Evidence for increase in sarcotubular calcium stores mediated by cyclic 3′,5′-AMP. Circ. Res.25, 429–438 (1969)
Fransden, E.K., Krishna, G.: A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sciences18, 529–542 (1976)
Friedmann, N.: Effects of glucagon and cyclic-AMP on ion fluxes in the perfused liver. Biochim. Biophys. Acta274, 214–225 (1972)
George, W.J., Polson, J.B., O'Toole, A.G., Goldberg, N.D.: Elevation of guanosine 3′,5′-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc. Nat. Acad. Sci. USA66, 398–403 (1970)
Gillette, R.W., McKenzie, G.O., Swanson, M.H.: Modification of the lymphocyte response to mitogens by cyclic AMP and cyclic GMP. J. Reticuloendoth. Soc.16, 289–299 (1974)
Gilman, A.G.: A protein binding assay for adenosine 3′,5′-cyclic monophosphate. Proc. Nat. Acad. Sci. USA67, 305–312 (1970)
Goldberg, N.O., O'Dea, R.F., Haddox, M.K.: Cyclic GMP. In: Advances in cyclic nucleotide research (Greengard, P., and Robison, G.A., eds.), Vol. 3, pp. 155–223. New York: Raven Press 1973
Graham, R.C., Karnovsky, M.J.: The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: Ultrastructural cytochemistry by a new technique. J. Histochem. Cytochem.14, 291–302 (1966)
Herrmann-Erlee, M.P.M.: A parathyroid-like action of dibutyryl cyclic adenosine 3′,5′-monophosphate on the explanted embryonic mouse radius. Calcif. Tiss. Res.4 (supplement), 70–72 (1970)
Ignarro, L.J., George, W.J.: Mediation of immunologic discharge of lysosomal enzymes from human neutrophils by guanosine 3′,5′-monophosphate. J. Exp. Med.140, 225–238 (1974)
Johnson, L.D., Hadden, J.W.: Cyclic GMP and lymphocyte proliferation: Effects of DNA-dependent RNA polymerase I and II activities. Biochem. Biophys. Res. Commun.66, 1498–1505 (1975)
Mason, T.E., Phifer, R.F., Spicer, S.S., Swallow, R.A., Dreskin, R.B.: An immunoglobulin-enzyme bridge method for localizing tissue antigens. J. Histochem. Cytochem.17, 563–569 (1969)
Matthews, J.L., Martin, J.H.: Intracellular transport of calcium and its relationship to homeostasis and mineralization: An electron microscope study. Am. J. med.50, 589–597 (1971)
McGuire, J.L., Marks, S.C.: The effects of parathyroid hormone on bone cell structure and function. Clin. Orthopaed.100, 392–405 (1974)
Murad, F., Brewer, H.B., Jr., Vaughn, M.: Effect of thyrocalcitonin on adenosine 3′,5′-cyclic phosphate formation by rat kidney and bone. Proc. Nat. Acad. Sci. USA65, 446–453 (1970)
Nagata, N., Sasaki, M., Kimura, N., Nakane, K.: Effects of porcine calcitonin on the metabolism of calcium and cyclic AMP in rat skeletal tissue in vivo. Endocrinology97, 527–535 (1975)
Nagata, N., Kimura, N., Sasaki, M., Nakane, K., Tanaka, Y.: Localization of cell groups sensitive to parathyroid hormone and calcitonin in rat skeletal tissue. Biochim. Biophys. Acta421, 218–227 (1976)
Park, H.Z., Talmage, R.V.: Relation of endogenous parathyroid secretion to3H-cytidine incorporation into bone cells. Endocrinology80, 552–560 (1967)
Peck, W.A., Carpenter, J., Messinger, K., DeBra, D.: Cyclic 3′,5′ adenosine monophosphate in isolated bone cells: Response to low concentrations of parathyroid hormone. Endocrinology92, 692–697 (1973)
Prince, W.T., Berridge, M.J., Rasmussen, H.: Role of calcium and adenosine 3′,5′-cyclic monophosphate in controlling fly salivary gland secretion. Proc. Nat. Acad. Sci. USA69, 553–557 (1972)
Rao, L.G., Brunette, D.M., Heersche, J.N.M.: PTH-response after long-term culture and sub-culture of cells from newborn rat calvaria. J. Dent. Res.55, B303 (1976)
Roberts, W.E.: Cell population dynamics of periodontal ligament stimulated with parathyroid extract. Am. J. Anat.143, 363–370 (1975)
Roberts, W.E., Chamberlain, J.G.: Scanning electron microscopy (SEM) of the cellular and vascular elements of rat periodontal ligament (PDL). J. Dent. Res.55, B165 (1976)
Rodan, S.B., Rodan, G.A.: The effect of parathyroid hormone and thyrocalcitonin on the accumulation of cyclic adenosine 3′,5′-monophosphate in freshly isolated bone cells. J. Biol. Chem.249, 3068–3074 (1974)
Rodan, G.A., Bourret, L.A., Harvey, A., Mensi, T.: Cyclic AMP and cyclic GMP: Mediators of the mechanical effects on bone remodeling. Science189, 467–469 (1975)
Shanfeld, J., Shapiro, I., Davidovitch, Z.: The measurement of adenosine 3′,5′-monophosphate in bone. Anal. Biochem.66, 450–459 (1975)
Smith, D.M., Johnston, C.C., Jr.: Cyclic 3′,5′-adenosine monophosphate levels in separated bone cells. Endocrinology96, 1261–1269 (1975)
Soderling, T.R., Hickenbottom, V.P., Reimann, E.M., Hunkeler, F.L., Walsh, D.A., Krebs, E.G.: Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3′,5′-monophosphate-dependent protein kinases. J. Biol. Chem.245, 6317–6328 (1970)
Talmage, R.V., Cooper, C.W., Park, H.Z.: Regulation of calcium transport in bone by parathyroid hormone. Vitamins and Hormones28, 103–140 (1970)
Voorhees, J.J., Stawiski, M., Duell, E.A.: Increased cyclic GMP and decreased cyclic AMP levels in the hyperplastic, abnormally differentiated epidermis of psoriasis. Life Sciences13, 639–653 (1973)
Walsh, D.A., Perkins, J.P., Krebs, E.G.: An adenosine 3′,5′-monophosphate-dependent protein kinase from rabbit skeletal muscle. J. Biol. Chem.243, 3763–3765 (1968)
Weisbrode, S.E., Capen, C.C., Nagode, L.A.: Effects of parathyroid hormone on bone of thyroparathyroidectomized rats. Am. J. Path.75, 529–536 (1974)
Weissmann, G., Goldstein, I., Hoffstein, S., Tsung, P.K.: Reciprocal effects of cAMP and cGMP on microtubule-dependent release of lysosomal enzymes. Ann. N.Y. Acad. Sci.253, 750–762 (1975)
Wicks, W.D.: Tyrosine-α-ketoglutarate transaminase: Induction by epinephrine and adenosine 3′,5′-cyclic phosphate. Science160, 997–998 (1968)
Wicks, W.D.: Regulation of protein synthesis by cyclic AMP. In: Advances in cyclic nucleotide research. (Greengard, P. and Robison, G.A., eds.), Vol. 4, pp. 335–438. New York: Raven Press 1974
Zimmerman, T.P., Chu, L.C., Winston, M.S.: A more sensitive radioimmunoassay (RIA) for cyclic GMP (cG). Fed. Proc.34, 231 (1975)
Zurier, R.B., Hoffstein, S., Weissmann, G.: Mechanisms of lysosomal enzyme release from human leukocytes: I. Effect of cyclic nucleotides and colchicine. J. Cell Biol.58, 27–41 (1973)
Zurier, R.B., Weissmann, G., Hoffstein, S., Kammerman, S., Tai, H.H.: Mechanisms of lysosomal enzyme release from human leukocytes: II. Effects of cAMP and cGMP, autonomic agonists and agents which affect microtubule function. J. Clin. Invest.53, 297–309 (1974)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davidovitch, Z., Montgomery, P.C. & Shanfeld, J.L. Cellular localization and concentration of bone cyclic nucleotides in response to acute PTE administration. Calc. Tis Res. 24, 81–91 (1977). https://doi.org/10.1007/BF02223300
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02223300