Summary
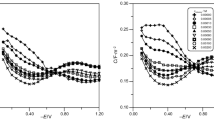
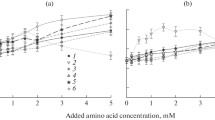
The influence of the ionic strength of the medium on the adsorption of bacteriophage T 2 to the surfaces of a mercury drop** electrode on one hand and ofbacteria E. coli B on the other hand was studied. The adsorption on the mercury surface was determined by measurement of the differential capacity of the electrode double layer, the adsorption to bacteria was estimated from the decrease of free phage particles in a bacterial suspension with time. The adsorption to the mercury electrode increases with increasing ionic strength of the medium, but adsorption to the surface of bacteria increases at first, has a maximum at concentrations between 0,1 to 0,5 M and decreases with further increase of ionic strength. The decrease of adsorption of phage to the bacterial surface is assumed to be caused by the blocking of specific sites on the bacterial surface by adsorbed ions which sterically prevent the adsorption of the phage. Such specific sites are not present on the electrode surface, therefore adsorption increases further with increasing ionic strength probably due to the neutralization of surface charges of the phage and of the electrode. The saturated surface-concentration of the phageγ s was calculated from the dependence of the differential capacity on the concentration. It is concluded fromγ s value obtained that the phage particles are scattered with wide intervals on the electrode surface with a degree of coverage of approximately 1∶40.
Similar content being viewed by others
Abbreviations
- DNA:
-
deoxyribonucleic acid
- N:
-
Avogadro number
Literature
Adams, M. H.: Bacteriophages. New York: Intersci. Publ. 1959.
Bendet, I. J., L. G. Swaby, andM. A. Lauffer: Biochim. biophys. Acta (Amst.)25, 252 (1957).
Cummings, D. J., andL. M. Kozloff: J, molec. Biol.5, 50 (1962).
Delahay, P., andCh. W. Tobias (ed): Advances in Electrochemistry and Electrochemical Engineering, p. 17. New York: Intersci. Publ. 1961.
Dirkx, J.: Contribution à l'étude de la fixation des bactériophages sur les bactéries sensibles. Acta Medica Belgica (Bruxelles) Separatum (1963).
Frédericq, P.: C. R. Soc. Biol. (Paris)146, 327 (1952).
Frumkin, A. N., andB. B. Damaskin: Dokl. Akad. Nauk SSSR129, 862 (1959).
Fujimura, R., andP. Kaesberg: Biophys. J.2, 433 (1962).
Garen, A.: Biochim. biophys. Acta (Amst.)14, 163 (1954).
Herčík, F.: Biophysik der Bakteriophagen, S. 76. Berlin: Deutscher Verl. Wiss. 1959.
Herriott, R. M., andJ. L. Barlow: J. gen. Physiol.40, 809 (1957).
Lorenz, W., F. Möckel, andW. Müller: Z. phys. Chem. NF25, 145 (1960).
Maites, L.: Polarographic Techniques. New York: Intersoi. Publ. 1955.
Melik-Gajkazjan, V. I.: Zh. fizič. chimii26, 560 (1952).
—: Zh. fizič. chimii26, 1184 (1952).
—Miller, I. R.: J. molec. Biol.3, 229 (1961).
—, andC. D. Grahame: J. Amer. chem. Soc.78, 3577 (1956).
Paleček, E.: J. molec. Biol.20, 263 (1966).
Puck, T. T., A. Garen, andJ. Cline: J. exp. Med.93, 65 (1951).
—, andL. J. Tolmach: Arch. Biochem.51, 229 (1954).
Staniewski, R., M. Kowalski, andK. Gorzkowska: Acta microbiol. pol.12, (3), 184 (1963).
Stent, G. S.: Molecular biology of bacterial viruses, p. 88. San Francisco: W. H. Freeman 1963.
Tolmach, L. J., andT. T. Puck: J. Amer. chem. Soc.74, 5551 (1952).
Vetterl, V.: Collection Czech. Chem. Comm.31, 2105 (1966).
Volkenshtein, M. V.: Structure and physical properties of molecules, p. 497. Moskva: Izdatelstvo akademii nauk SSSR 1955.
Author information
Authors and Affiliations
Additional information
The authors wishes to express their gratitude to the late Prof.Ferdinand Hercík, director of the Institute of Biophysics, for the initiation of this work and stimulating interest. The authors are also indebted to Dr. J.Koudelka for his kind gift of phage T 2 sample and to Dr. M.Vízdalová for her valuable comments during preparation of this article.
Rights and permissions
About this article
Cite this article
Brabec, V., Vetterl, V. Adsorption of bacteriophage on bacterial surface and on the surface of Hg drop** electrode. Biophysik 3, 361–376 (1967). https://doi.org/10.1007/BF01228320
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01228320