Summary
-
1.
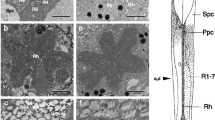
We have discovered a previously unreported visual mutant of the blowfly,Calliphora erythrocephala. It shows a reduced or absent visual performance, e.g., in escape and optomotor behavior. The effects of this mutation on the ultrastructure were studied by electron microscopy (Figs. 3–8) and on electrophysiological function, by intracellular recordings (Figs. 1 and 2).
-
2.
The genetic basis of this spontaneous mutation was studied by test crosses of mutant and wild-type flies. The defect appears to be in an autosomal recessive gene (Table 1).
-
3.
Of the mutant stock studied soon after eclosion (n = 18) 35% shows optomotor reactions, whereas only 6% studied in later life (n = 240) shows any optomotor behavior.
-
4.
The absence of the receptor potentials in photoreceptor cells is not directly associated with structural disorders in the early life of these mutant flies, but several types of degenerative changes are manifested in the retinular cells later on. The optomotorically blind specimens have normal (about −60 mV) resting membrane voltages but no detectable receptor cell voltage response to light, indicating a block in phototransduction. The spectral and polarization sensitivities of optomotor-positive flies are normal (Fig. 2).
-
5.
At the beginning of degeneration the number of lysosomes in the receptor cells is increased compared with normal flies, but their number as well as that of other components of the cell interior decrease later on. During the progression of the degeneration, the rhabdomeres shrink while the mitochondria swell and disintegrate (Figs. 6–8).
-
6.
The blocking of phototransduction is proposed to lead to disturbance of the turnover of the rhabdomeres and finally to degeneration of the receptor cells.
Similar content being viewed by others
Abbreviations
- norpA :
-
no receptor potential A
- ora :
-
outer rabdomere absent
- rdgA :
-
retinal degeneration A
- rdgB :
-
retinal degeneration B
- rER:
-
rough endoplasmic reticulum
- sER:
-
smooth endoplasmic reticulum
- rpa :
-
receptor potential absent
References
Alawi AA, Jennings V, Grossfield J, Pak WL (1972) Phototransductionmutants ofDrosophila melanogaster. In: Arden GB (ed) The visual system: Neurophysiology, biophysics and their clinical applications. Plenum, New York, pp 1–21
Benzer S (1973) Genetic dissection of behavior. Sci Am 229:2–15
Blondeau J, Heisenberg M (1982) The three-dimensional optomotor torque system ofDrosophila melanogaster. J Comp Physiol 145:321–329
Burkhardt D (1962) Spectral sensitivity and other response characteristics of single visual cells in the arthropod eye. Symp Soc Exp Biol 16:86–109
Carlson SD, Stark WS, Chi C (1984) Rapid light induced degeneration of photoreceptor terminals inrdgB mutantDrosophila. Invest Ophthalmol Visual Sci Suppl “ARVO 84”
Hardie RC (1985) Functional organization of the fly retina. In: Ottoson D (ed) Progress in sensory physiology 5. Springer, Berlin Heidelberg New York, pp 1–79
Harris WA, Stark WS (1977) Hereditary retinal degeneration inDrosophila melanogaster. A mutant defect associated with the phototransduction process. J Gen Physiol 69:261–291
Harris WA, Stark WS, Walker JA (1976) Genetic dissection of the photoreceptor system in the compound eye ofDrosophila melanogaster. J Physiol 256:415–439
Heisenberg M (1979) Genetic approach to a visual system. In: Autrum H (ed) Vision in invertebrates (Handbook of sensory physiology vol. VII/6A). Springer, Berlin Heidelberg New York, pp 665–679
Hirosawa K, Hotta Y (1982) Morphological analysis of photoreceptor membranes in mutantDrosophila eyes. In: Hollyfield JG (ed) The structure of eye. Elsevier/North Holland, pp 45–53
Hotta Y, Benzer S (1970) Genetic dissection of theDrosophila nervous system by means of mosaics. Proc Natl Acad Sci USA 67:1156–1163
Inoue H, Yoshioka T, Hotta Y (1985) A genetic study of inositol triphosphate involvement in phototransduction usingDrosophila mutants. Biochem Biophys Res Commun 132:513–519
Inoue H, Yoshioka T, Hotta Y (1988) Membrane associated phospholipase-C ofDrosophila retina. J Biochem 103:91–94
Järvilehto M (1985) The eye: Vision and perception. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon, Oxford New York Toronto, pp 355–429
Järvilehto M, Moring J (1976) Spectral and polarization sensitivity of identified retinal cells of the fly. In: Zeltler F, Weiler R (eds) Neural principles in vision. Springer, Berlin Heidelberg, pp 214–226
Järvilehto M, Zettler F (1971) Localized intracellular potentials from pre- and postsynaptic components in the external plexiform layer of an insect retina. Z Vergl Physiol 75:422–440
Järvilehto M, Zettler F (1973) Electrophysiological-histological studies on some functional properties of visual cells and second order neurons of an insect retina. Z Zellforsch 136:291–306
Järvilehto M, Weckström M, Kouvalainen E, Harjula R (1986) Ultrastructural and functional pathology of the compound eye of the retinal degeneration mutant. Proc Scand Electron Microsc: 17
Johnson MA, Frayer KL, Stark WS (1982) Characteristics ofrdgA: Mutants with retinal degeneration inDrosophila. J Insect Physiol 28:233–242
Laughlin SB (1981) Neural principles in the peripheral visual system of invertebrates. In: Autrum H (ed) Vision in invertebrates (Handbook of sensory physiology, vol VII/6B). Springer, Berlin Heidelberg New York, pp 133–280
Lo M-VL, Pak WL (1981) Light-induced pigment granule migration in the retinular cells ofDrosophila melanogaster: Comparison of wild type with ERG defective mutants. J Gen Physiol 77:155–175
Meyertholen EP, Stein PJ, Williams MA, Ostroy SE (1987) Studies of theDrosophila norpA phototransduction mutant. II. Photoreceptor degeneration and rhodopsin maintenance. J Comp Physiol A 161:793–798
Pak WL (1975) Mutations affecting the vision ofDrosophila melanogaster. In: King RC (ed) Handbook of genetics, vol 3. Plenum, New York, pp 703–733
Payne R (1986) Phototransduction by microvillar photoreceptors of invertebrates: mediation of a visual cascade by inositol triphosphate. Photobiochem Photobiophys 13:373–397
Schwemer J (1985) Turnover of photoreceptor membrane and visual pigment in invertebrates. In: Stieve H (ed) The molecular mechanism of photoreception. Springer, Berlin Heidelberg New York, pp 303–326
Stark WS, Carlson SD (1984) Blue and ultraviolet light induced damage to theDrosophila melanogaster Meigen (Diptera: Drosophilidae). J Insect Morphol Embryol 14:243–254
Stark WS, Sapp R (1987a) Development and senescence of thenorpA (no receptor potential) mutant ofDrosophila. Invest Ophthalmol Visual Sci Suppl “ARVO 87”
Stark WS, Sapp R (1987b) Ultrastructure of the retina ofDrosophila melanogaster: The mutantora (outer rhabdomere absent) and its inhibition of degeneration inrdgB (retinal degeneration B). J Neurogenetics 4:227–240
Stark WS, Sapp R, Hartman CR (1986) Retinal degeneration and membrane cycling inrdgB mutantDrosophila: Interaction with mutants which block the receptor potential and the lysosomal enzyme acid phosphatase. Int Congr of Eye Research in Nagoya
Strausfeld NJ (1971) The organization of the insect visual system (Light microscopy), I. Projections and arrangements of neurons in the lamina ganglionaris of Diptera. Z Zellforsch 121:377–441
Torkkeli P, Weckström M, Kouvalainen E, Järvilehto M (1987) Retinal degeneration mutation in blowfly: functional and ultrastructural analysis. Neurosci 22 [Suppl]: 1240
Williams DS (1982) Rhabdom size and photoreceptor membrane turnover in a muscoid fly. Cell Tissue Res 226:629–639
Wilson MJ, Ostroy SE (1987) Studies of theDrosophila norpA phototransduction mutant. I. Electrophysiological changes and the offsetting effect of light. J Comp Physiol A 161:785–791
Yoshioka T, Inoue H, Hotta Y (1983) Defective phospholipid metabolism in the retinular cell membrane ofnorpA. (no receptor potential) visual transduction mutants ofDrosophila. Biochem Biophys Res Commun 111:567–573
Yoshioka T, Inoue H, Hotta Y (1984) Absence of diglyceride kinase activity in the photoreceptor cells ofDrosophila mutants. Biochem Biophys Res Commun 119:389–395
Yoshioka T, Inoue H, Hotta Y (1985) Absence of phosphatidylinositol phosphodiesterase in the head of aDrosophila visual mutant,norpA (no receptor potential A). J Biochem 97:1251–1254
Young RW (1977) Visual cell renewal systems and the problem of retinitis pigmentosa. In: Landers MB, Wolbarsht MY, Dowling JE, Laties AM (eds) Pigmentosa: clinical implications of current research. Plenum, New York, pp 93–113
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Torkkeli, P., Weckström, M., Kouvalainen, E. et al. Morphological and functional characteristics of arpa (receptor potential absent) visual mutant of the blowfly (Calliphora erythrocephala). J. Comp. Physiol. 165, 333–341 (1989). https://doi.org/10.1007/BF00619352
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00619352