Conclusions
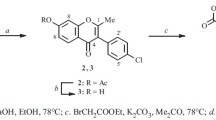
A number of natural isoflavones and their structural analogs have been synthesized by improved ethyl orthoformate and dimethylformamide methods. It has been possible to use the dimethylformamide method for the first time for the synthesis of 5,7-dihydroxyisoflavones. Two new isoflavones have been synthesized: 7-methoxyisoflavone and 5,7-dihydroxy-3′,4′-dimethoxyisoflavone.
Similar content being viewed by others
Literature cited
V. V. Mezheritskii and G. N. Dorofeenko, Zh. Obshch. Khim.,40, 2459 (1970).
S. A. Kagal et al., Tetrahedron Lett.,14, 593 (1962).
V. A. Bandyukova, Rast. Res.,8, No. 2, 283 (1972).
F. M. Dean, Naturally Occurring Oxygen Ring Compounds, Butterworth's Scientific Publications, London (1963), p. 366.
Additional information
Rostov Scientific-Research Institute of Physical and Organic Chemistry at Rostov State University, Pyatigorsk Pharmaceutical Institute. Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 160–162, March–April, 1974.
Rights and permissions
About this article
Cite this article
Dorofeenko, G.N., Shinkarenko, A.L., Kazakov, A.L. et al. Synthesis of natural isoflavones and their structural analogs. Chem Nat Compd 10, 173–174 (1974). https://doi.org/10.1007/BF00563606
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00563606