Summary
The 3-D distribution of atrial and ventricular isomyosins is analysed in tubular chicken hearts (stage 12+ to 17 (H/H)) using antibodies specific for adult chicken atrial and ventricular myosin heavy chains, respectively.
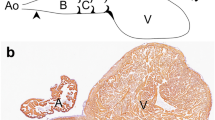
At stage 12+ (H/H) all myocytes express the atrial isomyosin; furthermore, all myocytes except those originally situated in the dorsolateral wall of the sinu-atrium coexpress the ventricular isomyosin as well. Moreover, it appears that recently incorporated myocardial cells at both ends of the heart tube start with a coexpression of both isomyosins. From stage 14 (H/H) onwards a regional loss of expression of one of either isomyosins is observed in the atrial and ventricular compartment. In this way the single isomyosin expression types that are characteristic for the adult working myocardium of the atria and ventricles arise. So, the isomyosin expression patterns are, unexpectedly, hardly useful to discriminate the different heart parts of the tubular heart. The ventricle, defined by its adult type of isomyosin expression, is even not detectable before stage 14 (H/H). Interestingly, interconnected coexpression areas, which may be precursor conductive tissues, are still present at stage 17 (H/H) in the outflow tract, the ventricular trabeculae, the atrio-ventricular transitional zone and in the sinuatrium.
The pattern of isomyosin coexpression was found to correlate with a peristaltoid contraction and a slow conduction velocity, whereas single expression areas correlate with a synchronous contraction and a relatively fast conduction velocity.
The possible implications of the changing isomyosin pattern for the differentiation of the tubular myocardium, in particular in relation to the development of the conductive tissues, will be discussed.
Similar content being viewed by others
Abbreviations
- H/H :
-
staged according to the classification of Hamburger and Hamilton (1951)
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1983) In: Molecular Biology of the Cell. Garland Publishing, New York, London
Argüello C, De la Cruz MV, Gómez CS (1975) Experimental study of the formation of the heart tube in the chick embryo. J Embryol Exp Morphol 33:1–11
Argüello C, Alanis J, Pantoja O, Valenzuela B (1986) Electrophysiological and ultrastructural study of the atrioventricular canal during the development of the chick embryo. J Mol Cell Cardiol 18:499–510
Benninghoff A (1923) Über die Beziehungen des Reizleitungssystems und der Papillarmuskeln zu der Konturfaser des Herzschlauches. Anat Anzeiger 57: Ergänzungsheft, 185–208
Boucek RJ, Murphy Jr WP, Paff GH (1959) Electrical and mechanical properties of chick embryo heart chambers. Circ Res 7:787–793
Burnette WN (1981) “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203
Castro-Quezada A, Nadal-Ginard B, De la Cruz MV (1972) Experimental study of the formation of the bulboventricular loop in the chick. J Embryol Exp Morphol 27:623–637
De Groot IJM, Hardy GPMA, Sanders, E, Los JA, Moorman AFM (1985) The conducting tissue in the adult chicken atria: A histological and immunohistochemical analysis. Anat Embryol 172:239–245
De Groot IJM, Sanders E, Visser SD, Lamers WH, De Jong F, Los JA, Moorman AFM (1986) Isomyosin expression in the develo** chicken atria: A marker for the development of conductive tissue? Anat Embryol, in press
De Haan RL (1961) Differentiation of the atrioventricular conducting system of the heart. Circulation 24:458–470
De Jong F (1984) (abstract) New transparent 3-D reconstruction techniques and their application to analysis of heart morphogenesis. Acta Morphol Neerl Scand 22:160
De la Cruz MV, Gómez CS, Arteaga MM, Argüello C (1977) Experimental study of the development of the truncus and the conus in the chick embryo. J Anat 123:661–686
González-Sánchez A, Bader D (1984) Immunochemical analysis of myosin heavy chains in the develo** chicken heart. Dev Biol 103:151–158
González-Sánchez A, Bader D (1985) Characterization of a myosin heavy chain in the conductive system of the adult and develo** chicken heart. J Cell Biol 100:270–275
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hirota A, Sakai T, Fujii S, Kamino K (1983) Initial development of conduction pattern of spontaneous action potential in early embryonic precontractile chick heart. Dev Biol 99:517–523
Ho E, Shimada Y (1978) Formation of the epicardium studied with the scanning electron microscope. Dev Biol 66:579–585
Johnstone PN (1971) Studies on the physiological anatomy of the embryonic heart. Thomas, Springfield, Illinois, USA
Kim Y, Yasuda M (1980) Development of the cardiac conducting system in the chick embryo. Anat Histol Embryol (Berlin) 9:7–20
Kuro-O M, Tsuchimochi H, Ueda S, Takaku F, Yazaki Y (1986) Distribution of cardiac myosin isozymes in human conduction system. Immunohistochemical study using monoclonal antibodies. J Clin Invest 77 (2):340–348
Lieberman M, Paes de Carvalho A (1965a) The electrophysiological organization of the embryonic chick heart. J Gen Physiol 49:351–364
Lieberman M, Paes de Carvalho A (1965b) The spread of excitation in the embryonic chick heart. J Gen Physiol 49:365–379
Lieberman M (1970) Physiologic development of impulse conduction in embryonic cardiac tissue. Am J Card 25:279–284
Manasek FJ (1969) Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol 22:233–248
Manasek FJ (1970) Histogenesis of the embryonic myocardium. Am J Cardiol 25:149–168
Paff GH, Boucek RJ (1962) Simultaneous electrocardiograms and myograms of the isolated atrium, ventricle and conus of the embryonic chick heart. Anat Rec 142:73–80
Paff GH, Boucek RJ, Harrell TC (1968) Observations on the development of the electrocardiogram. Anat Rec 160:575–582
Patten BM (1922) The formation of the cardiac loop in the chick. Am J Anat 30:373–397
Patten BM, Kramer TC (1933) The initiation of contraction in the embryonic chick heart. Am J Anat 53:349–375
Patten BM (1956) The development of the sinoventricular conduction system. Univ Mich Med Bull 22 (1):1–21
Sanders E, Moorman AFM, Los JA (1984) The local expression of adult chicken heart myosins during development. I. The three days embryonic chicken heart. Anat Embryol 169:185–191
Sanders E, De Groot IJM, Geerts WJC, De Jong F, Van Horssen AA, Los JA, Moorman AFM (1986) The local expression of adult chicken heart myosins during development. II. Ventricular conducting tissue. Anat Embryol 174:187–193
Sartore S, Pierobon-Bormioli S, Schiaffino S (1978) Immunohistochemical evidence for myosin polymorphism in the chicken heart. Nature (London) 274:82–83
Schwartz K, Lecarpentier Y, Martin JL, Lompré AM, mercadier JJ, Swynghedauw B (1981) Myosin isoenzymic distribution correlates with speed of myocardial contraction. J Mol Cell Cardiol 13:1071–1075
Sweeney LJ, Clark WA, Zak R, Manasek FJ (1983) (abstract) Myosin heavy chain expression during cardiac embryogenesis in the chick. J Cell Biol 97:51a nr 193
Sweeney LJ, Clark WA, Umeda PK, Zak R, Manasek FJ (1984) Immunofluorescence analysis of the primordial myosin detectable in embryonic striated muscle. Proc Natl Acad Sci USA 81:797–800
Sweeney LJ, Nag AC, Eisenberg B, Manasek FJ, Zak R (1985) Developmental aspects of cardiac contractile proteins. Basic Res Cardiol 80 (Suppl 2):123–137
Van Mierop LHS (1967) Location of pacemaker in chick embryo heart at the time if initiation of heartbeat. Am J Physiol 212:407–415
Virágh GZ, Challice CE (1983) The development of the early atrioventricular conduction system in the embryonic heart. Can J Physiol Pharmacol 61:775–792
Wenink ACG (1976) Development of the human cardiac conducting system. J Anat 121:617–631
Whalen RG (1985) Myosin isoenzymes as molecular markers for muscle physiology. J Exp Biol 115:43–53
Zhang Y, Shafiq SA, Bader D (1986) Detection of a ventricularspecific myosin heavy chain in adult and develo** chicken heart. J Cell Biol 102:1480–1484
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Jong, F., Geerts, W.J.C., Lamers, W.H. et al. Isomyosin expression patterns in tubular stages of chicken heart development: a 3-D immunohistochemical analysis. Anat Embryol 177, 81–90 (1987). https://doi.org/10.1007/BF00325291
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00325291