Abstract
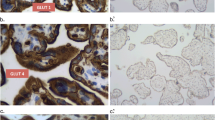
The localization has been investigated of the isoforms GLUT1, GLUT3 and GLUT4 of glucose transporter proteins as well as of insulin receptors. Fetal membranes (n=10) were examined by immunohistochemical methods at the light and electron microscopic levels using mono- and polyclonal antibodies. In all amnion epithelial cells, GLUT1 and GLUT3 antibodies were bound to the apical membrane. Very rarely the GLUT1 antibody also immunostained the basolateral membrane and reacted weakly with the endomembrane system and membranes of the lateral cell protrusions. Fibroblasts reacted with the antibodies against GLUT1, GLUT4 and insulin receptor, whereas they were labelled only in one case with GLUT3 antibody. Cytotrophoblast cells were only stained with antibodies against GLUT1 and GLUT3. Antibodies against GLUT4 only reacted with fibroblasts in the membranes. On amnion epithelial cells, weak immunoreactivity with insulin receptor antibodies was detected only at the electron microscopic level. The data indicate: (1) GLUT1 is located on all cells of the amnion, whereas GLUT3 is present in detectable amounts only on amnion epithelial cells and cytotrophoblast; (2) GLUT1 and GLUT3 on amnion epithelial cells are predominantly located on the apical surface; (3) GLUT4 and insulin receptors are not regularly expressed. We suggest that amnion epithelial cells cover their basal glucose requirements from the amniotic fluid and not from the maternal circulation.
Similar content being viewed by others
References
Allen SR, Smith CH (1992) Glucose transport in the human placenta: GLUT1 and GLUT3 glucose transporter protein localization in syncytiotrophoblast microvillous and basal membranes. 39th Annual Meeting of the Society for Gynecological Investigation, March 1992, Atlanta, Ga., USA, p 281
Bell G, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S (1990) Molecular biology of mammalian glucose transporters. Diabetes Care 13:198–208
Benirschke K, Kaufmann P (1990) Placental membranes. In: Benirschke K, Kaufmann P (eds) Pathology of the human placenta, 2nd edn. Springer, New York, pp 130–179
Bergmann JS, Carney DH (1982) Receptor-bound thrombin is not internalized through coated pits in mouse embryo cells. J Cell Biochem 20:247–253
Desoye G (1988) The human placenta in gestational diabetes. In: Weiss PAM, Coustan D (eds) Gestational diabetes. Springer, Wien, pp 72–86
Desoye G, Hartmann M, Blaschitz A, Dohr G, Kohnen G, Kaufmann P (1992) Immunohistochemical evidence that the majority of insulin receptors in human term placenta is located on endothelial cells. Placenta 13:A13
Forsayeth J, Caro CF, Sinha MK, Maddoux BA, Goldfine ID (1987) Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci USA 84:3448–3451
Gautier N, Alengrin F, Ponzio G, Rossi B, Dolais-Kitabgi J (1986) Etude des fonctions du recepteur de l'insuline a l'aide d'anticorps monoclonaux anti-recepteur. Ann Endocrinol 47:215
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubule of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–306
Haspel HC, Rosenfeld MG, Rosen OM (1988) Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem 263:398–403
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Hüter J, Grossmann D, Brunnengräber M (1970) Paraplacentarer Stoffaustausch an menschlichen Eihäuten. Geburtshilfe Frauenheilkd 30:245–255
Hüter J, Dreher F, Müller V (1972) Chorioamnialer Transfer zwischen Gravida und Fetus. Geburtshilfe Frauenheilkd 32:169–175
James DE, Brown R, Navarro J, Pilch PF (1988) Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333:183–185
Jones CJ, Hartmann M, Blaschitz A, Desoye G (1993) Ultrastructural localization of insulin receptors in human placentae. Am J Reprod Immunol (in press)
Mazurkiewicz-Kilczenska D (1971) Hexosamines and their biosynthesis in human amnion in vitro. Am J Obstet Gynecol 110:355–357
Morgan DO, Roth RA (1986) Map** surface structures of the human insulin receptor with monoclonal antibodies: localization of main immunogenic regions to the receptor kinase domain. Biochemistry 25:1364–1371
Morriss FH Jr, Boyd RDH (1988) Placental transport. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York, pp 2043–2083
Nelson DM, Smith RM, Jarett L (1978) Non-uniform distribution and grou** of the insulin receptors on the surface of human placental syncytial trophoblast. Diabetes 27:530–538
Plough T, Stallknecht BM, Pedersen O, Kahn BB, Ohkuwa T, Vinten J, Galbo H (1990) Effect of endurance training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. Am J Physiol 259:E778-E786
Schmidt W (1965) Über den paraplacentaren, fruchtwassergebundenen Stofftransport beim Menschen. I. Histochemische Untersuchungen der in den Eihäuten angereicherten Stoffe. Z Anat Entwickl Gesch 124:321–334
Schmidt W (1992) The amniotic fluid compartment: the fetal habitat. Adv Anat Embryol Cell Biol 127:1–100
Schmidt W, Pfaller K, Schwarzfurtner H (1982) Licht-und elektronenmikroskopische Untersuchungen an den Eihäuten. I. Amnion und Zwischenschicht. Zentralbl Gynäkol 104:385–396
Schwartz AL, Forster CS, Smith PA, Liggins PC (1977) Human amnion metabolism. I. In vitro maintenance. Am J Obstet Gynecol 127:470–474
Scoggin WA (1976) Fetal membrane dynamics. Am J Obstet Gynecol 124:825–831
Shafrir E, Diamant YZ (1979) Regulation of placental enzymes of the carbohydrate and lipid metabolic pathway. Ciba Found Ser 63:161–179
Shepherd PR, Gould GW, Colville CA, McCoid SC, Gibbs EM, Kahn BB (1992) Distribution of GLUT3 glucose transporter protein in human tissues. Biochem Biophys Res Commun 188:149–154
Sinha AA (1971) Ultrastructure of human amnion and amniotic plaques of normal pregnancy. Z Zellforsch 122:1–14
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1992) Localization of erythrocyte/HepG2-type glucose transporter (GLUT1) in human placental villi. Cell Tissue Res 267:407–412
Varndell M, Polak JM (1986) Electron microscopical immunocytochemistry. In: Polak JM, Van Noorden S (eds) Immunocytochemistry, modern methods and applications. Wright, Bristol, pp 146–167
Wang T (1980) Amniotic epithelium in diabetes mellitus: Light- and electron microscopic examination. Virchows Arch Pathol Anat Histopathol 387:185–191
Weiss PAM, Hofmann H, Winter R, Pürstner P, Lichtenegger W (1985) Amniotic fluid glucose values in normal and abnormal pregnancies. Obstet Gynecol 65:333–339
Weser H, Kaufmann P (1978) Lichtmikroskopische und histochemische Untersuchungen an der Chorionplatte der reifen menschlichen Placenta. Arch Gynäkol 225:15–30
Wolf HJ, Schmidt W (1991) Histochemical examinations on the carbohydrate metabolism in the fetal membranes. Acta Histochem 91:3–11
Wolf HJ, Schmidt W, Drenckhahn D (1991) Immunocytochemical analysis of the cytoskeleton of the human amniotic epithelium. Cell Tissue Res 266:385–389
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wolf, H.J., Desoye, G. Immunohistochemical localization of glucose transporters and insulin receptors in human fetal membranes at term. Histochemistry 100, 379–385 (1993). https://doi.org/10.1007/BF00268936
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00268936