Abstract
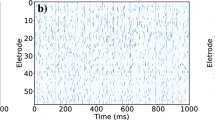
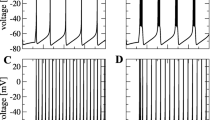
Electrophysiological properties of neurons as the basic cellular elements of the central nervous system and their synaptic connections are well characterized down to a molecular level. However, the behavior of complex noisy networks formed by these constituents usually cannot simply be derived from the knowledge of its microscopic parameters. As a consequence, cooperative phenomena based on the interaction of neurons were postulated. This is a report on a study of global network spike activity as a function of synaptic interaction. We performed experiments in dissociated cultured hippocampal neurons and, for comparison, simulations of a mathematical model closely related to electrophysiology. Numeric analyses revealed that at a critical level of synaptic connectivity the firing behavior undergoes a phase transition. This cooperative effect depends crucially on the interaction of numerous cells and cannot be attributed to the spike threshold of individual neurons. In the experiment a drastic increase in the firing level was observed upon increase of synaptic efficacy by lowering of the extracellular magnesium concentration, which is compatible with our theoretical predictions. This “on-off” phenomenon demonstrates that even in small neuronal ensembles collective behavior can emerge which is not explained by the characteristics of single neurons.
Similar content being viewed by others
References
Abeles M (1991) Corticonics. Cambridge University Press, Cambridge
Amit DJ (1989) Modeling brain function. Cambridge University Press, Cambridge
Amit DJ, Gutfreund H, Sompolinsky H (1985) Spin-glass models of neural networks. Phys Rev A 32: 1007–1018
Bartlett WP, Banker GA (1984) An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. II. Synaptic relationships. J Neurosci 4: 1954–1965
Bekkers JM, Stevens CF (1991) Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA 88: 7834–7838
Benson DL, Watkins FH, Steward O, Banker G (1994) Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol 23: 279–295
Buhmann J, Schulten K (1987) Influence of noise on the function of a “physiological” neural network. Biol Cybern 56: 313–327
Czeh G, Somjen GG (1989) Changes in extracellular calcium and magnesium and synaptic transmission in isolated spinal cord. Brain Res 486: 274–285
Fletcher TL, Cameron P, Camilli P de, Banker G (1991) The distribution of synapsin I and synaptophysin in hippocampal neurons develo** in culture. J Neurosci 11: 1617–1626
Griffiths RB (1972) Rigorous results and theorems. In: Domb C, Green MS (eds) Phase transitions and critical phenomena, vol I. Academic, London, pp 7–109
Habets AMMC, Van Dongen AMJ, Van Huizen F, Corner MA (1987) Spontaneous neuronal firing pattern in fetal rat cortical networks during development in vitro: a quantitative analysis. Exp Brain Res 69: 43–52
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100
Hopfield JJ (1982) Neural networks and physical systems with emergent computational abilities. Proc Natl Acad Sci USA 79: 2554–2558
Hopfield JJ, Tank DW (1985) “Neural” computation of decisions in optimization problems. Biol Cybern 52: 141–152
Ising E (1925) Beitrag zur Theorie des Ferromagnetismus. Z Phys 31: 253–261
Kleinfeld D, Sompolinsky H (1988) Associative neural network model for the generation of temporal patterns: theory and application to central pattern generators. Biophys J 54: 1039–1051
Matthiessen HP, Schmalenbach C, Müller HW (1989) Astroglia-released neurite growth-inducing activity for embryonic hippocampal neurons is associated with laminin bound in a sulphated complex and free fibronectin. Glia 2: 177–188
Mody I, Lambert JDC, Heinemann U (1987) Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol 57: 869–888
Mountcastle VB (1957) Modality and topographic properties of single neurons of cat's sensory cortex. J Neurophysiol 20: 408–434
Palm G (1980) On associative memory. Biol Cybern 36: 19–32
Purves D, Riddle DR, LaMantia AS (1992) Iterated patterns of brain circuitry (or how the cortex gets spots). Trends Neurosci 15: 362–368
Scheff SW, Price DA (1993) Synapse loss in the temporal lobe in Alzheimer's disease. Ann Neurol 33: 190–199
Segal M, Barker JL (1984) Rat hippocampal neurons in culture: voltage-clamp analysis in inhibitory synaptic connections. J Neurophysiol 52: 500–515
Sejnowski T, Koch C, Churchland P (1988) Computational neuroscience. Science 241: 1299–1306
Siebler M, Koller H, Stichel CC, Müller HW, Freund HJ (1993) Spontaneous activity and recurrent inhibition in cultured hippocampal networks. Synapse 14: 206–213
Szentagothai J (1975) The “module-concept” in cerebral cortex architecture. Brain Res 95: 475–496
Szentagothai J (1983) The modular architectonic principle of neural centers. Rev Physiol Biochem Pharmacol 98: 11–61
Watson PG (1972) Surface and size effects in lattice models. In: Domb C, Green MS (eds) Phase transitions and critical phenomena, vol II. Academic, London, pp 101–159
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rose, G., Siebler, M. Cooperative effects of neuronal ensembles. Exp Brain Res 106, 106–110 (1995). https://doi.org/10.1007/BF00241360
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00241360